7th Multilateral Initiative on Malaria (MIM) Pan African Conference – 2018: Day 5
Friday, 20th April 2018
Published: 05/12/2018
This report is brought to you by the MESA Correspondents Shehu Shagari Awandu, Manuela Runge, Helena Martí Soler, and Camila Damasceno. Senior editorial support has been facilitated by Ingeborg van Schayk.
THEMES: THEMES: Basic Science | Drug Resistance | Epidemiology | Health Systems | Product Development | Vaccines | Vector Control
MESA Correspondents bring you cutting-edge coverage from the 7th Multilateral Initiative on Malaria (MIM) Pan African Malaria Conference
Day 5: Friday, April 20th
Plenary session:
The plenary speakers were introduced by Professor Oumar Gaye from the University Cheikh Anta Diop, Senegal, and President of the MIM Organising Committee.
“When will we have a Licenced malaria vaccine?”
Prof Adrian Hill, Director of the Jenner Institute at Oxford University, UK, explained that a deployable multistage-subunit vaccine that combines sporozoite stages, liver stages, and mosquito stages is currently in development.
Such a multistage vaccine approach would provide higher efficacy, protect the vaccine against parasite escape mutations and would allow for host heterogeneity in immune responses. Besides, the vaccine would also be advantageous for both disease prevention and transmission reduction. While the most advanced malaria vaccine to date, the RTS,S vaccine, had demonstrated up to 36% efficacy over 3-4 years in children 5-17 months of age, the immunity waned rapidly and would need a booster dose. He stressed that the next phase of the RTS,S would need to consider the safety issues, such as the increased all cause female mortality in vaccinees, before the new target licensure in endemic areas by 2024. He opined that while the quest for whole sporozoite vaccine had shown promising results and could even be licensed before the RTS,S vaccine, several challenges still remain, notably the high costs of manufacture, the need for cold chain storage, and the modest efficacy in African adults.
Prof Hill showed the results of a subunit vaccine, the R21. The R21 has shown 100% efficacy in mice and is highly immunogenic for only circumsporozoite (CSP) repeat region, in contrast to RTS,S, which is highly immunogenic for both CSP repeat and hepatitis B surface antigens. Phase I/II trials of the R21/Matrix-M have shown good safety profile in >1,500 subjects, and the vaccine is less costly to manufacture and has better potential in younger infants. The R21 clinical development plan that includes trials in endemic African populations are under design with target licensure in 2023. Prof. Hill further shared results of P. falciparum RH5 and its improvement RH5.2, which has shown the first blood stage efficacy as assessed by controlled human malaria infections (CHMI). The goal for RH5.2 vaccine development program involves improving its immunogenicity, the quality of vaccine-induced IgGs and identifying additive or ideally synergistic antigen combinations. On transmission blocking vaccines, he outlined some of the leading parasite candidates expressed both in the vector and in the host.
He concluded his talk by focussing on prime target vaccination that requires priming intramuscular immunisation followed by an intravenous boost to target the liver. The approach induces high levels of resident memory T cells in the liver, with a markedly increased preclinical malaria vaccine efficacy.
“Challenges and Perspective in Ending Malaria – Drug Resistance”
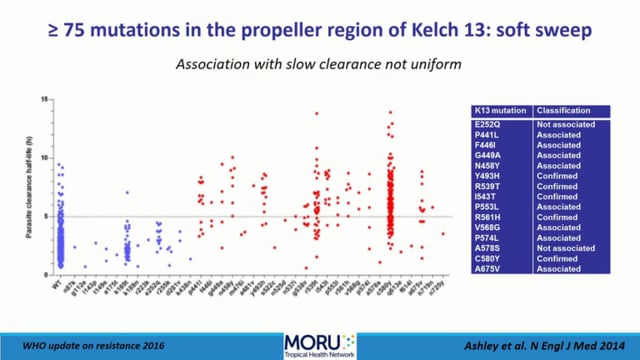
Despite the serious emerging and real threat of drug resistant parasites, the malaria community does not need to panic, according to Prof Christopher Plowe, the Director of Duke Global Health Institute, Duke University, USA. It is not gloom and doom as the artemisinin-based combination therapies (ACTs) still remain efficacious in most of the Greater Mekong Sub-Region, the epicentre of reported artemisinin resistance. In addition, in Africa artemisinin resistance has not taken hold yet and the genetic background of ACT resistant “strains” is vulnerable to recombination after spreading to higher transmission zones.
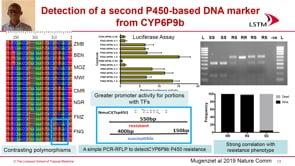
In the past 20 years, drug resistance research has included identifying the genetic determinants of resistance to chloroquine, antifolates, artemisinins, and several ACT partner drugs. The time from initial appearance of resistance to identifying resistance markers has shortened from decades for chloroquine to a few years for artemisinins, to having candidate markers in hand for new drugs before they are even deployed. This progress is attributable in part to technological advances in genome sequencing, as well as genetic and in vitro manipulation of malaria parasites. Better and earlier integration of these basic sciences with field epidemiology has also accelerated the identification and validation of resistance markers.
Genomic epidemiological evidence that a “tsunami” of artemisinin resistance was both spreading transnationally out of Cambodia and emerging independently in multiple locations contributed to the decision to launch an aggressive malaria elimination campaign in the Greater Mekong Sub-Region. Mathematical modelling of resistance and of interventions aiming to mitigate resistance has also influenced malaria drug treatment and prevention policies.
While questions remain on how to monitor resistance when malaria becomes rare, Prof Plowe shared details of a novel ultrasensitive method that uses small volumes of blood and is amenable for large scale molecular surveillance monitoring of drug resistance, especially in eliminating countries. He emphasized that for maximum impact, drug resistance research must continue to cross barriers between basic and applied sciences and between the cultures of research and of program implementation and policymaking. An “all hands on deck” approach that calls for interdisciplinary research that integrates not just genomics and epidemiology but vector biology, health economics, social, environmental, and political sciences, and healthcare inequalities and health systems research is necessary.
Reported by Shehu Shagari Awandu and Helena Martí Soler
Symposium: “Environmental Compliance Concerns and Solutions that Arise from Malaria Control via Indoor Residual Spraying (IRS)”
Indoor residual spraying is a widely used intervention for malaria control. The safe handling, deployment and adequate disposal of insecticides require local solutions according to international recommendations.
Dr Yemane Yihdago, of AIRS Ghana, Abt Associates, presented their experience with the recycling of organophosphate insecticide bottles used during indoor residual (IRS) campaigns. The project aimed to free storage space for the following campaign-season and to avoid risks to humans, animals, and environmental contamination. As a result, final products such as garbage bins and pavement blocks confirmed as non-hazardous, are being produced in at least 12 countries where IRS is conducted. This project managed to achieve savings on transport and incineration and showed a positive impact on the environment.
Dr Peter Chandonait, Director of Environmental Compliance and Safety of the PMI AIRS project showed how secondary package cardboard cartons of insecticides from IRS campaigns in Rwanda are recycled into greeting cards. The boxes are not insecticide contaminated and are donated to Cards from Africa, a fair-trade and socially committed company in Rwanda.
Tahina Masihelison, Environmental Compliance Officer from Abt Associates, presented a mobile Soak Pit for environmental compliance during IRS campaigns. He stated that the building and maintenance of facilities for IRS equipment cleaning in remote areas is difficult, thus innovative technologies are needed. Mobile Soak Pits were designed as a transportable, easy to install and effective replacement for fixed pits, ideal for travelling teams. This approach enhances the safety for IRS operators with minimum environmental impact.
The last presentation illustrated how countries can deal with large quantities of unused insecticides. Ethiopia had previously had to deal with tons of obsolete DDT, years after identifying mosquito resistance to DDT back in 2010. The management of large amounts of DDT in Ethiopia was presented by Yohannes Ameha, Senior Environmental Health Expert from the Ethiopian Ministry of Environment. Due to risks and costs of other disposal options, Ethiopia opted for transboundary transportation to a licensed incinerator. The project resulted in the safe disposal of 119 tons of DDT and contaminated waste.
Reported by Camila Damasceno
Symposium: ”Progress in Malaria Transmission Blocking Vaccine Development”
The progress of candidate transmission blocking vaccines (TBV) development with the focus on Plasmodium falciparum antigens Pfs230, Pfs25 and Pfs48/45, was discussed. Results from recent clinical trials as well as approaches to measuring TBV activity in mosquitoes were presented in the perspective of bringing TBVs into use in Africa.
Dr Ashley Birkett, from the PATH’s Malaria Vaccine Initiative, set the stage for the rest of the talks and also gave the final presentation, highlighting past progress and current challenges. Remarkable progress has been made in overcoming challenges, such as scalability of manufacturing and formulation of priority antigens, pre-clinical antibodies and their predictive value, and the generation of human proof of concept data. However, more needs to be done to bridge “lab” and “field” transmission measures. Humanized monoclonal antibodies may be a promising approach for bridging that gap when addressing field efficacy in the perspective of heterologous protection, and durability of protection for at least two years.
Dr Mamadou Coulibaly from MRTC/USTTB, presented novel approaches to measure the activity of transmission blocking vaccines in the field. Lab evaluation methods of TBV activity include standard membrane feeding-assays (SMFA) and direct membrane feeding assay (DMFA) with direct skin feeding (DSF) being tested as a new approach in the field. In Mali, already 9,000 DSFs have been conducted since 2011. Community consent and safety precautions in mosquito rearing were identified as critical aspects of the studies. DSF was found to be safe and well tolerated but with low infectivity. Work is on-going to optimise the approach. In order to do so, experimental huts in near natural settings are used as well as community malaria transmission studies.
Prof Robert Sauerwein, from the Radboud University Medical Center, talked about preclinical development of Pfs48/45 vaccine candidates. The development of R0-6C looks promising, with established upstream production of research cell banks and downstream purification. Work is on-going for the optimization of immunogenicity. In the coming years, a cell bank for manufacturing according to good manufacturing practice as well as potency and toxicity studies will be established, and clinical trials will start in healthy volunteers.
Dr Patrick Duffy from the Laboratory of Malaria Immunology and Vaccinology, NIAID/NIH presented results on US trials of Pfs25 and Pfs230 vaccine candidates. They compared functional antibody activities of Pfs25 and Pfs230 alone as well as in combination. The results have shown no difference on the activity of Pfs25 and Pfs230 conjugates in mice but differences in non-human primates (NHP). Moreover, Pfs230D1 was found to be superior to Pfs25 for inducing functional activity and the combination with Pfs25 may not enhance the activity. Overall, the clinical trials are addressing key translational goals and are contributing to bridging the gap between the lab and the field.
Prof Issaka Sagara from the Malaria Research and Training Center (MRTC); USTTB Bamako, has investigated Pfs25 and Pfs230 and their combination in field trials in Mali since 2013. Immunogenicity and activity were assessed using SMFA, while DSF was used to explore activity further. Results showed that Pfs25 and Pfs230 were both well tolerated with antibody responses in almost all subjects (Pfs230 greater responses than Pfs25).
TBVs will require mass administrations to achieve herd immunity in order to reduce the incidence of infections in a community at high levels. According to the experts, in combination with other tools, they could potentially play an essential role in malaria elimination and eventually eradication.
Reported by Manuela Runge
This blog was written by Camila Damasceno (Independent Consultant), Manuela Runge (Swiss TPH), Shehu Shagari Awandu (Radboud UMC) and Helena Marti Soler (ISGlobal) as part of the MESA Correspondent program, and is cross-posted on the MESA website and Malaria World.
Published: 05/12/2018
This report is brought to you by the MESA Correspondents Shehu Shagari Awandu, Manuela Runge, Helena Martí Soler, and Camila Damasceno. Senior editorial support has been facilitated by Ingeborg van Schayk.
THEMES: Basic Science | Drug Resistance | Epidemiology | Health Systems | Product Development | Vaccines | Vector Control