9th PAMCA Annual Conference and Exhibition – 2023: Day 3
Wednesday, 20th September 2023
Published: 20/09/2023
This report is brought to you by the MESA Correspondents Akua Obenewaa Danquah Yirenkyi, Ashu Fred Ayukarah, Augustino Mmbaga, Helga D.M. Saizonou, Julius Ichodo Odero, Ndey Bassin Jobe, Temesgen Ashine, and Diane Leslie Nkahe. Senior editorial support has been facilitated by Zawadi Mboma and Billy Tene.
THEMES: THEMES: Vector Control
MESA Correspondents bring cutting-edge coverage from the 9th PAMCA Annual Conference and Exhibition “Reorienting surveillance and management in the context of emerging threats of disease vectors”.
Plenary Talk 5 – Malaria, eradication and the role of vector control
Helen Jamet (Bill and Melinda Gates Foundation – BGMF, United States) began her presentation by discussing the three strategic principles of the BMGF for malaria eradication in Sub-Saharan Africa which are (i) reducing the burden of malaria, (ii) investing in next generation surveillance systems, and (iii) mitigating resistance to drugs and insecticides. The BMGF budget for malaria is $1.6B and 23% of it goes towards vector control research and development (R&D). The BMGF key priority areas for vector control R&D includes (i) new insecticide active ingredients (AI’s) to fight against resistance (e.g., dual AI nets and attractive targeted sugar baits), and (ii) genetic based vector control methods (e.g., gene drive). She highlighted PAMCA’s resourcefulness in helping BMGF to collaborate with African researchers towards a common goal of making the continent free of vector borne diseases. Jamet informed, the BMGF remains committed to supporting and investing in initiatives that are geared towards contributing to malaria elimination in Sub-Saharan Africa. She concluded by encouraging the PAMCA chapters and research institutions to provide recommendations for the future of PAMCA’s core mandates.
Parallel Scientific Session 11 – LLINS, IRS and insecticide resistance management
Session Chair: Julien Z.B. Zahouli; Co-chair: Mame Niang
Julien Z.B. Zahouli (Swiss Centre for Scientific Research – CSRS, Ivory Coast) compared the efficacy of washed and unwashed PermaNet Dual®, PermaNet® 3.0 and PermaNet® 2.0, with unwashed nets as control, using experimental huts, then laboratory cone and tunnel bioassays in Tialassé, Ivory Coast. Unwashed and 20 times washed bed nets were exposed to wild Anopheles gambiae s.l. Results indicated the inhibition in mortality and blood-feeding for both unwashed and washed nets was higher with PermaNet® Dual compared to the other nets and the control. To conclude, Zahouli stated that the deltamethrin-chlorfenapyr coated PermaNet Dual® represents a promising tool in the fight against pyrethroid-resistant Anopheles vectors.
Eliningaya J. Kweka (Tanzania Plant Health and Pesticides Authority, Tanzania) presented research work on the potential new tool, In2Care® EaveTubes, a plastic ventilation sheet with mosquito killing netting to address the now established challenge of insecticide resistance. This tool works by funneling human-scented air when indoors through the eaves tube to lure mosquitoes and when they are blocked, the special static coating in the netting binds mosquitoes to it. To evaluate the efficacy of this promising insecticide candidate, the team carried out a pilot study at the Kagera Sugar Limited premises in Tanzania. The study results showed that the In2care EaveTube caused an overall 58 % reduction in malaria mosquitoes and up to 75% in peak season. A similar study carried out in Ivory Coast and published in the Lancet (link) showed a reduction of malaria cases by 47% in houses treated with EaveTubes. The presenter concluded by requesting a scale up of this new tool, which is still awaiting WHO review, to manage insecticide resistance.
Patrick Tungu (National Institute for Medical Research, Tanzania) introduced his talk describing pyrethroid resistance in Tanzania. The study was about experimental hut and community (Phase III) cluster randomized indoor residual spraying evaluation trials of VECTRON™ T500 against malaria vectors in Tanzania. The mortality to VECTRON™ T500 recorded during experimental hut trials in Moshi was non-inferior to that of Actellic® 300CS, while the ability of VECTRON™ T500 to reduce vector density during the community randomized trial conducted in Muheza was non-inferior to that of Fludora® Fusion. The efficacy of VECTRON™ T500 on mud and concrete walls lasted 12 months post spraying against insecticide susceptible and resistant An. gambiae s.s., confirming the long-lasting residual efficacy of VECTRON™ T500. Patrick closed by announcing the validation of VECTRON™ T500 by the WHO in March 2023.
Moussa B.M. Cisse (Laboratory of Applied Molecular Biology – LBMA, Mali) centered his presentation around the need for the deployment of two sets of insecticides Fludora® Fusion 562.5 WP-SB and Ficam®VC against pyrethroid-resistant Anopheles gambiae s.l in Mali. He highlighted the serious challenge faced by core vector intervention, IRS, that shifted their focus towards the two novel insecticides. They conducted Phase three trials collecting data through prokopack aspiration, human landing catches, capture with exit, and performed dissections. They also carried out WHO cone test and CDC bottle bioassays on the mosquitoes of interest. The study results showed that both Fludora® Fusion 562.5 WP-SB and Ficam®VC were effective in the control of pyrethroid-resistant strains An. gambiae s.l. with residual efficacy of 12 months and 5-6 months respectively. To address this urgent need, the insecticides under consideration should be deployed to combat the challenge of insecticide resistance that is threatening to disrupt progress in malaria vector control in Mali.
Dunia Munyakanage (Malaria and Other Parasitic Diseases Division – MOPDD, Rwanda), on behalf of Elias Niyituma (Abt Associates, Rwanda) started his presentation by informing the audience that in 2013, Rwanda adopted an insecticide resistance management strategy due to widespread resistance to pyrethroid insecticides, IRS and ITN being the core vector intervention tools. Based on malaria endemicity and insecticide resistance data, a new generation of insecticide was introduced in 2017 and new PBO nets in 2020. Through a study, the new tools (IRS with Actellic 300CS and new PBO) were evaluated against Interceptor G2 (IG2) nets. IRS, IG2, and PBO nets were deployed in Ngoma, Karongi, and Kicukiro districts respectively. The results showed that entomological inoculation rates were lower after the intervention in all three districts. However, the reduction was higher in the IRS and PBO net districts than in the IG2 net district. Also, IRS with Actellic 300CS was more efficient against An. gambiae than PBO nets, thus the need to use new formulations of IRS for effective management of malaria hotspots.
Parallel Scientific Session 12 – Vector bionomics: vector biology, ecology, taxonomy and population genetics
Session Chair: Penny Hancock; Co-chair: Rita Mwima
Luciano Michaël Tantely (Institut Pasteur of Madagascar, Madagascar) did a nationwide inventory of Anopheles species available in Madagascar. With the rise of malaria cases, it was necessary to extend entomological studies by examining potential vector transmitters different from the well-studied Anopheles gambiae s.l species. The study was carried out from February to December 2019. It was found in April that, along with An. gambiae s.l, an abundance of An. coustani and An. squamosus; both attracted to humans, were recorded and corroborated with the higher prevalence of Plasmodium sp. in that month.
Rocco D’Amato (Innovation, Genomics, Genetics and Biology Hub – Polo GGB, Italy), following the WHO guidelines to evaluate genetically engineered mosquitoes (GEM), used large cages to evaluate a double-sex-targeting gene drive as well as an anti-drive to prevent population collapse and compared the results to those observed in small cages. He found that in both cages’ population suppression was effective after the drive insertion, and the anti-drive insertion was also able to inhibit the drive spread. Nevertheless, more fluctuations were observed in gene drive and anti-drive frequency in large cages highlighting the importance of large cage studies prior to field trials.
Esinam Abla Akorli (Noguchi Memorial Institute for Medical research, Ghana) studied the ecological factors associated with the presence of Microsporidia MB in mosquito breeding sites. Her study first highlighted the presence of Microsporidia MB in Anopheles gambiae and Anopheles coluzzii from Ghana, with the prevalence being higher in males. Further investigation of the breeding sites led her to understand that rice fields were compatible with the growth of this bacteria, while factors such as turbidity, salinity, copper, zinc, and manganese presence in breeding sites were negatively correlated with MB presence. This work informs on ecological factors that need consideration if MB is to be considered as symbiont-mediated malaria control.
Penny Hancock (Imperial College London, United Kingdom) used data from a pesticide spray catch model conducted in 4 villages in Burkina Faso for 6 years to simulate field data trial sets for gene drive implementation. Since gene drive is a method set for imminent trial, it is important to statistically estimate its effects along with how much release will be needed to achieve 90% suppression. The number of mosquitoes collected, their spatial distribution, and the mosquito species collected were used to construct a Bayesian spatiotemporal model for fitting mosquito count. The simulation model shows that to detect 50% suppression, it is necessary for five villages to receive the same drive simultaneously, with this power decreasing if only one mosquito species is targeted. This shows that time series data can inform the design of field trials to detect vector suppression.
Parallel Scientific Session 13: Vector surveillance: surveillance systems, community-based surveillance, epidemiology, disease control programs and global health
Session Chair: Joel C Mouatcho; Co-chair: Brenda Onyango
Gabriel O Kotewas (Ministry of Health – MoH, Kenya) presented their study on the effectiveness of indoor residual spraying (IRS) on malaria control in Kenya. They conducted a review of the malaria cases among children under five years in Rachuonyo North Sub County, Homa Bay County, from 2019 to 2022. Results showed malaria cases were recorded all year round with two major malaria peak seasons. During the four-year study, the highest number of malaria cases in Rachuonyo North were recorded in 2020 and the lowest number of cases were recorded in 2021. According to Kotewas, the reduction of malaria cases was largely associated with the use of IRS and insecticide-treated bed nets (ITNs) since 2018.
Rock Aikpon (Ministry of Health/National Malaria Control Programme – MoH/NMCP, Benin) presented a study on the effect of IRS interventions withdrawal on malaria transmission in two districts in Benin. Mosquitoes were collected between 2016 and 2018 using the human landing catch technique, identified first morphologically then using species PCR. ELISA tests were performed for sporozoite detection. Results revealed over 3-fold increase in vector abundance with Anopheles gambiae being the most predominant vector species. Moreover, they observed that vectors became more endophagic. This study reveals that the withdrawal of IRS confers vulnerability to the population with regards to malaria transmission.
Daniel Nguiffo Nguete (Centre for Research in Infectious Diseases – CRID, Cameroon) presented a study on the contribution of Plasmodium malariae to high levels of malaria transmission in a forest savannah transition area in Cameroon. They conducted entomological surveys to identify which vectors were most competent to transmit P. malariae. They also evaluated the dynamics of P. falciparum and P. malariae infection transmission in all age groups. The results of the study showed that the most abundant species was An. funestus followed by An. gambiae. Infections caused by P. malariae or co-infections of P. malariae and P. falciparum in humans were transmitted mainly by An. funestus. Also, children under the age of 5 had a significantly increased density of plasmodium compared to other age groups. Overall, P. falciparum infections were the most prevalent (43%) followed by co-infections of P. malariae and P. falciparum (17%). He concluded that additional interventions should be implemented to tackle P. malariae in malaria endemic countries.
Eric Ochomo (Kenya Medical Research Institute – KEMRI, Kenya and Centre for Global Health Research – CGHR, Kenya) presented the first detection of the invasive malaria vector, An. stephensi, in Kenya, which was discovered through molecular surveillance. The mosquitoes used in the study were collected from December 2022 to February 2023, and identified using (i) PCR, (ii) morphological identification, and (iii) additional verification of the results through amplicon sequencing. Ochomo concluded that although An. stephensi was detected in this analysis, it has not yet been incriminated as a vector of P. vivax in this study. Furthermore, surveillance of An. stephensi has been scaled up in many counties, led by the Kenya NMCP and PMI.
Joel C Mouatcho (KwaZulu-Natal Malaria Control Programme, South Africa) presented results of entomological surveillance carried out in KwaZulu-Natal in order to understand if residual malaria transmission is driven by outdoor or indoor biting vectors. Mosquitoes were collected outdoors and indoors using diverse collection techniques. Mosquitoes were also identified by morphology and PCR. Overall, results revealed that 97% of the mosquitoes were collected outdoors, more precisely around animal shelters. An. arabiensis was the most predominant vector species. This study highlights the likely implication of outdoor biting mosquitoes in residual transmission of malaria in KwaZulu-Natal.
Domonbabele François de Sales Hien (Research Institute of Health Sciences – IRSS, Burkina Faso) presented a study on the effects of the alkaloid ricinine on the capacity of An. gambiae and An. coluzzii to transmit P. falciparum. They assessed (i) the effect of alkaloid ricinine on the competence of mosquitoes to transmit P. falciparum, and (ii) the rate of oocyst development and sporozoite dissemination in salivary glands. Results showed that the consumption of nectar alkaloid ricinine could have effects on the phenotypic traits that govern malaria transmission. However, more studies need to be done to validate the efficacy of alkaloid ricinine as a novel control agent for malaria vectors.
Parallel Scientific Sessions 15: Vector surveillance: surveillance systems, community-based surveillance, epidemiology, disease control programs and global health
Session Chair: Alison M Reynolds; Co-chair: Joel Lutomiah
Mark Wamalwa (International Centre of Insect Physiology and Ecology – ICIPE, Kenya) presented a study titled ‘The Exigent Threat of the Alien Invasive Anopheles stephensi in Africa.’ This study aimed to assess the risk of malaria transmission in Africa as a result of An. stephensi invasion by using a mathematical model. An agent-based compartmental model (ABM) using a bottom-up modeling approach was used while considering parameters such as urbanization, vector bionomics, and climate change scenarios. Findings suggested a shift in the distribution of An. stephensi in space and time, and that temperature significantly affects mosquito population growth rates. Wamalwa concluded by insisting on the need for strengthening surveillance, adaptive, and integrated vector control strategies, and improving information exchange.
Joyce Nyirongo (Malaria Alert Centre – MAC, Malawi) presented a study that focused on detection of gametocytes in asymptomatic malaria infections using microscopy. They aimed to quantify the frequency of asymptomatic malaria infections in a study group of 146 participants who were screened for infection. The study’s findings revealed that 47% of gametocyte positive participants were between the ages of 6 and 15 years old, consistent with previous research that has identified school-aged children as the primary P. falciparum transmitters to mosquitoes. Notably, some participants had multiple positive smears and came from the same household. Nyirongo concluded by highlighting the significance of investigating individual and household-level infection clusters and the transmission potential of asymptomatic gametocyte carriers.
Thiery Nirina Jean Jose Nepomichene (Institut Pasteur of Madagascar, Madagascar) presented on the field assessment of resting boxes for the surveillance of malaria vectors in the Central Highlands of Madagascar. To compare the trapping effectiveness of the Resting Box (RB), a surveillance tool made from locally available materials was compared with Muirhead Thomson pits (MTPs) as a standard method. Although both RB and MTPs were able to catch eight different species of Anopheles mosquitoes, both anthropophilic and zoophilic vectors, and sporozoite-infected mosquitoes, RBs captured approximately 18 times less Anopheles than MTPs. Improvement of RB is required, as it would be cost-effective in assisting community-based entomological surveillance.
Jaelsa Mira Goncalves Moreira (Ministry of Health – MoH, Cape Verde) was excited to share that Cape Verde was now eligible to receive a WHO malaria elimination certificate. She stated that this was the third time in the last three decades that they had achieved zero malaria transmission status, recording zero local cases of malaria since 2018. On track to eliminate malaria and get certified as malaria-free, Moreira discussed the Ministry of Health’s plans to prevent the reintroduction of malaria into the country. She also emphasized the importance of funding and partnerships that provide standard operating procedures (SOPs) and entomological surveillance to prevent malaria reintroduction. The certification will demonstrate the country’s commitment to eliminating vector-borne diseases and will serve as an encouragement to other African countries that it is possible to defeat malaria.
Parallel Symposium Session 12 – Alternative high-potential vector control strategies to mitigate insecticide resistance and reduce the malaria burden
Organizer: Chouaibou S. Mouhamadou
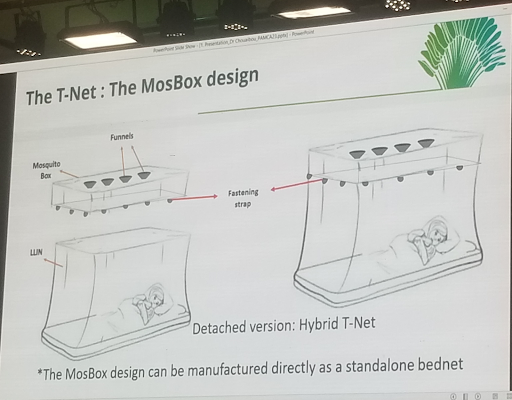
Chouaibou S. Mouhamadou (Swiss Centre for Scientific Research – CSRS, Ivory Coast) began by highlighting alternative tools that could complement existing malaria vector control tools. Mouhamadou then discussed the potential of a mosquito-trapping bednet (T-Net) to mitigate insecticide resistance. T-Net is a square-box design net with trapping funnels to be sewn on the top of the bednet. An experimental hut trial conducted in a high-resistance area (Tiassale) showed a 4-fold increase in mortality in untreated T-Net compared to Permanet 2.0. These findings also suggest an increased killing effect of ITNs when insecticide-treated T-nets are used. He added that this tool can limit insecticide-resistance selection pressure on mosquitoes, increase the killing effect of ITNs, and decrease the spread of resistance genes. He concluded by highlighting their goal, which was to generate data to demonstrate the public health value and community acceptability.
Cyrille Ndo (Center for Research for Infectious Disease – CRID, Cameroon) presented a study titled “Keeping the vector out: Re-introducing housing improvement as a sustainable approach to integrated vector control”. Ndo pointed out that we need an additional vector control tool that can curtail residual malaria transmission in areas where universal coverage of ITNs is not achieved, where ITNs show limited efficacy due to physical degradation and/or reduced bio-efficacy, and where people are exposed to mosquito bites before going to bed. Housing improvement is the better option. Evidence from field trials in Cameroon suggests that only by screening eaves with wire mesh, indoor mosquito density was reduced significantly; furthermore, there were 4.8- and 5.7-fold reductions in the indoor human biting rate and entomological inoculation rate, respectively. Ndo concluded that housing improvement has the potential to be integrated with other vector control strategies in malaria-endemic areas.
Karine Mouline (Research Institute for Development – IRD, France) presented on embedding malaria control and ecological balance in a one health approach using Ivermectin, a systemic insecticide that could help to manage insecticide resistance. Ivermectin, an endectocide with rapid mosquitocidal effects against Anopheles (2-3 days), works both in animals and humans. Therefore, treating animals with long-acting ivermectin formulations (LAIFs) may help reduce the population of zoophilic malaria vectors. She further added that avoiding ecological risks in already fragile environments and agro-ecosystems is imperative. Mouline further discussed that, to embed malaria control using LAIFs in a one-health approach, it is essential to consider the local entomological context, animal breeding practices, agricultural practices, multidisciplinary approaches, and local practices to mitigate environmental toxicity. Despite its potential for malaria vector control, the performance of this approach depends on the animal/human ratio and coverage. Lastly, she pointed out that the 50% lethal concentration (LC50) values for non-targeted species found in sub-Saharan Africa should be established.
Cyrille Ndo (Center for Research for Infectious Disease – CRID, Cameroon) presented the historical success and progress made so far in the research and development of the sterile insect technique (SIT), an alternative high-potential malaria vector control tool to control malaria vectors regardless of their resistance status. SIT involves rearing radio-sterilized male mosquitoes and then releasing them to mate with wild females. As these females do not produce any offspring, the vector population declines over time. He mentioned a few notable successes of this approach in insect pest control and highlighted the progress achieved in research and development with regard to SIT including the development of necessary equipment for mass rearing, sterilizing, sex separation, and female elimination. He concluded by insisting that despite significant strides that have been made over the years, some steps must be completed before its implementation.
Parallel Symposium Session 16 – Controlling Emergent Anopheles stephensi in Ethiopia and Sudan (CEASE)
Organizer: Alison M. Reynolds, PhD
Adane Iyasu (Jimma University, Ethiopia) presented on the distribution and characteristics of An. stephensi in Ethiopia. They studied the routes of invasion of this invasive species, and explored the most effective vector control strategies that can be used to combat its further spread. The entomological surveillance was conducted in 26 sites. The immature mosquitoes were collected using standard dippers and the adult mosquitoes were collected using CDC light traps and mechanical aspirators (Prokopack aspirator). The results of their study showed that six (6) sites were positive breeding habitats for An. stephensi: 89.3% of the pooled immature samples that were screened and genotyped were confirmed to be An. stephensi. Also, they found that the most common blood meal sources for the collected adult An. stephensi were animals.
Hmooda Toto Kafy (Federal Ministry of Health, Sudan) presented on the entomological surveillance of An. stephensi in Sudan. Their study objective was to identify the route of invasion of An. stephensi and its current and potential distribution. The entomological surveillances were conducted in 61 selected sites (10 houses from each site). The study revealed that seven sites had positive breeding habitat for An. stephensi. Most of the anopheles mosquito larvae collected from indoor and outdoor water containers were An. stephensi. However, adult An. stephensi density per house was very low in the majority of positive sites. Furthermore, the study also found that there is a tendency for adult An. stephensi to rest outdoors rather than indoors.
Martin Donnelly (Liverpool School of Tropical Medicine, United Kingdom) presented on the diversity, population structure, and demography of An. stephensi in Sudan. They investigated (i) signatures of insecticide resistance, and (ii) the impact of An. stephensi invasion processes on our ability to use genomic data to guide operational decision making and for understanding how to control the vector. They observed the presence of sub-populations in different states with no sign of insecticide resistance selection. Results on diversity, runs of homozygosity and complicated structures vs native ranges equally suggested invasion from different sites.
Temesgen Ashine (Armauer Hansen Research Institute, Ethiopia) presented a case control study which will assess the impact of An. stephensi on urban malaria transmission in Metchara, Ethiopia. To this end, they will select accessible areas with ongoing malaria transmission where An. stephensi is present for the study. Study participants will be recruited from health facilities into the case group if they have a fever and positive microscopy/ rapid diagnostic test (RDT) for plasmodium parasite, and into the control group if they have a negative result. Home visits to participants will be conducted 48 hours after enrolment for the collection of malaria risk factor data and entomological surveillance.
This report is brought to you by the MESA Correspondents Akua Obenewaa Danquah Yirenkyi, Ashu Fred Ayukarah, Augustino Mmbaga, Helga D.M. Saizonou, Julius Ichodo Odero, Ndey Bassin Jobe and Temesgen Ashine, with the support of a former correspondent Leslie Diane Nkahe. Senior editorial support has been facilitated by Zawadi Mboma & Billy Tene.
Published: 20/09/2023
This report is brought to you by the MESA Correspondents Akua Obenewaa Danquah Yirenkyi, Ashu Fred Ayukarah, Augustino Mmbaga, Helga D.M. Saizonou, Julius Ichodo Odero, Ndey Bassin Jobe, Temesgen Ashine, and Diane Leslie Nkahe. Senior editorial support has been facilitated by Zawadi Mboma and Billy Tene.
THEMES: Vector Control