Last Updated: 08/04/2024
WHO review of malaria vaccine clinical development
Published: 16/03/2024
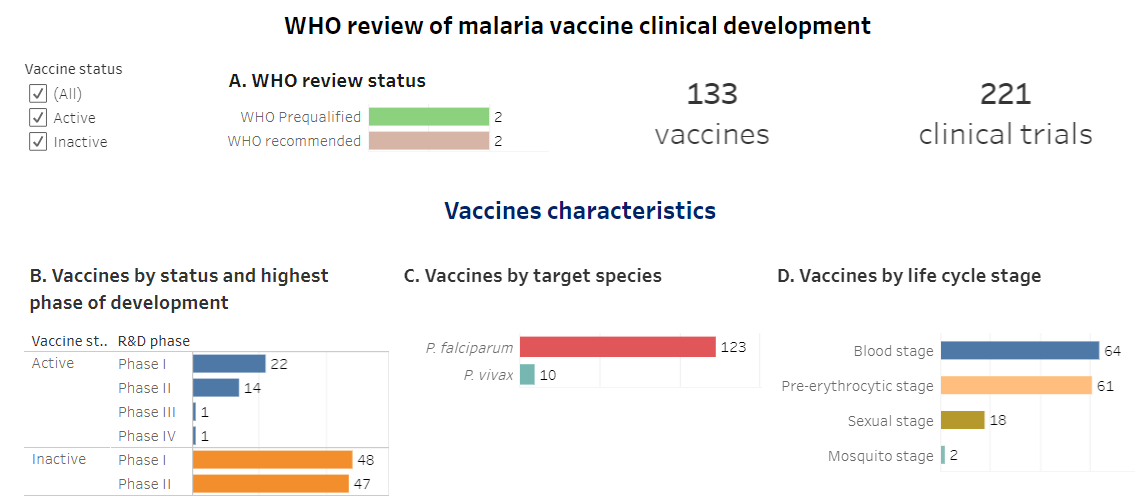
WHO analyzed the pipeline of malaria vaccines that were in phase I-IV of clinical development (as of December 2023). Products are reported by vaccine status (active/inactive), highest phase of clinical development, target malaria species, target life cycle stage, vaccine platform, target antigen, and adjuvant. Trials are reported by countries where trials are conducted, trial status, WHO Region and income group. This online tool provides comprehensive information on malaria vaccine clinical development, vaccine characteristics, clinical trial characteristics, and list of vaccine candidates and trials.
Related Resources
Debug_words_related: vaccine clinical
Related Projects
Debug_words_related: vaccine clinical