ASTMH 2019, Oumar Gaye: “Drug efficacy, safety and evaluation of drug resistance in IPTp and SMC chemoprevention strategies”
Collaborator(s): Cheikh Anta Diop University (UCAD), Senegal
Published: 23/11/2019
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 68th ASTMH annual meeting in Maryland, November 2019
Title: “Drug efficacy, safety and evaluation of drug resistance in IPTp and SMC chemoprevention strategies”
Author: Oumar Gaye, Universite Cheikh Anta Diop, Senegal
Session information:
Symposium 122: Learning From Experience to Optimize Chemoprevention Strategies For Malaria
November 23, 2019, 10:15 AM – 12:00 PM, Maryland C (Ballroom Level)
Abstract:
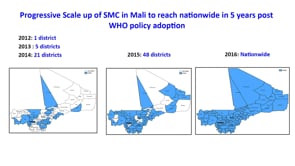
Intermittent preventive treatment in pregnancy (IPTp) and Seasonal Malaria Chemoprevention (SMC) are two of the WHO-recommended preventive chemotherapies that aim to prevent malarial illness in vulnerable populations. IPTp, implemented to date in 39 African countries, has been shown to reduce maternal malaria episodes and neonatal mortality, as well as other adverse effects of malaria in pregnancy. SMC could avert millions of cases and thousands of deaths among children aged 3 to 59 months living in areas of highly seasonal malaria transmission. This symposium addresses the latest advances in malaria chemoprevention and the challenges faced when translating and scaling up from the trials to the field. The two chemoprevention strategies have followed differing paths in terms of the research approaches to test their impact, the delivery mechanisms used and the policy-making process. This has influenced the coverage, the actions taken by the different actors and the uptake of the interventions in a different manner, and therefore, valuable lessons can be learned and applied to future strategies. IPTp and SMC will be used as examples to discuss the importance of fitting the strategies into the overall countries’ health systems and to think about what is needed to prepare interventions at large scale, and questions about safety and resistance will be raised, as well. The possibility of adding more drugs to the chemoprevention arsenal will be discussed in the last presentation, opening the floor to a final discussion about the impact and challenges when translating from trials to large scale in an effort to learn from experience and achieve the interventions’ full potential.