ASTMH 2019, Andrés Noé: “Novel methods to determine liver-stage malaria vaccine correlates of protection: kinetics, deep immune phenotyping and transcriptomics”
Collaborator(s): Jenner Institute, University of Oxford, United Kingdom
Published: 23/11/2019
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 68th ASTMH annual meeting in Maryland, November 2019.
Title: “Novel methods to determine liver-stage malaria vaccine correlates of protection: kinetics, deep immune phenotyping and transcriptomics”
Speaker: Andrés Noé, The Jenner Institute, University of Oxford, Oxford, United Kingdom
Session information:
November 23, 2019, 10:15 AM – 12:00 PM, Maryland B (Ballroom Level)
Abstract:
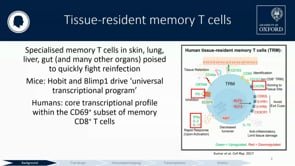
Protection from liver-stage malaria requires high numbers of CD8+ T cells to find and kill Plasmodium-infected cells. A new malaria vaccine strategy, Prime-Target Vaccination (PTV), involves sequential viral-vectored vaccination by intramuscular and intravenous routes to target cellular immunity to the liver. The efficacy of leading liver-stage malaria vaccine candidates in mice can be enhanced with this approach from 0-30% to 100%. PTV substantially increases the number of antigen-specific tissue-resident memory CD8+ T cells (TRMs) in the liver of mice. The first human phase I/IIa clinical trial of PTV efficacy against controlled human malaria infection is ongoing. We hypothesise that protection correlates to the magnitude of the TRM response elicited in the liver by vaccination. We have seen that cells with a TRM phenotype transiently appear in the blood of IV vaccinated individuals, which is consistent with data in animal models. Herein, we track the kinetics of these blood-derived TRMs with the expectation of identifying a correlate of vaccine protection against CHMI. Directly sampling the human liver safely is difficult but possible through liver fine needle aspiration (FNA). We safely obtained FNAs from 13 volunteers. Our project is the first-ever to perform deep immune phenotyping and transcriptomic evaluation of the intrahepatic effect of a new and promising liver-stage malaria vaccine strategy. As a means to identify correlates of liver-stage malaria protection, we obtained time-matched PBMC samples from the same volunteers who underwent FNA. We used flow cytometry and single-cell RNA sequencing on lymphocytes from the liver and blood to identify differentially expressed genes. This work provides clinical proof-of-concept of multiple novel methods to investigate pre-erythrocytic malaria vaccines, immunological evaluation of a malaria vaccine strategy by CHMI, and insights into correlates of protection after vaccination. A simple and reproducible correlate of protection would be particularly helpful in trials of liver-stage malaria vaccines as they progress to phase III, large-scale testing in African infants.
THEMES: Vaccines