ASTMH 2018, Pere Millat-Martínez: “Electrocardiographic safety evaluation of repeated courses of dihydroartemisinin-piperaquine for use in mass drug administration”
Collaborator(s): Barcelona Institute for Global Health (ISGlobal), Spain; Lihir Malaria Elimination Programme, Papua New Guinea
Countries: Papua New Guinea
Published: 31/10/2018
In collaboration with the poster presenters, MESA brings you this sneak-peak of poster 1834 from the 67th ASTMH annual meeting in New Orleans, October-November 2018
Title: “Electrocardiographic safety evaluation of repeated courses of dihydroartemisinin-piperaquine for use in mass drug administration”
Speaker: Pere Millat-Martínez, Lihir Malaria Elimination Programme, Londolovit, Lihir Island, NIP, Papua New Guinea/ ISGlobal, Barcelona, Spain
Abstract:
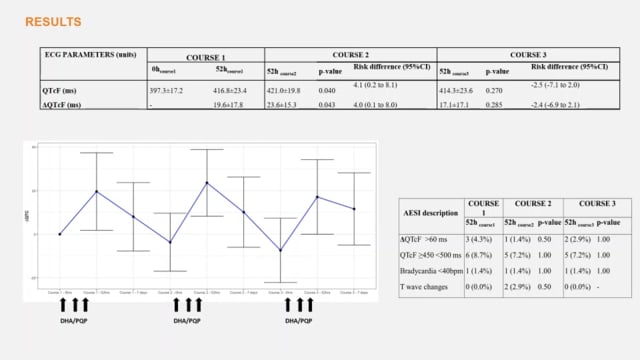
Repeated rounds of Mass Drug Administration (MDA) of antimalarial drugs is a modern approach to eliminate malaria. Dihydroartemisinin-Piperaquine (DHA/PQP) is an effective, well tolerated and long-acting antimalarial suitable for MDA. We assessed the cardiac safety of repeated treatment courses with DHA/PQP. METHODS: We conducted an interventional cohort study in Lihir Island, Papua New Guinea from Sept 21 to Dec 21, 2015. Consenting healthy volunteers were enrolled for repeated treatment with standard 3-day course of DHA/PQP 2.1/17.1 mg/Kg daily given monthly over 3 months. Eligible participants were healthy individuals aged 3 to 60 years. Triplicate twelve-lead ECG readings were conducted with an ELI 150 Cardiograph® at pre-dosing and 4h after the third dose of treatment with each monthly course. Interpretation of the tracings was independent and centralized at a cardiac laboratory. The primary endpoint was QTc prolongation from baseline to the 4h post-dosing ECG; safety was assessed comparing the difference in prolongation of the third course post-dosing ECG and the first course post-dosing ECG. Of 84 enrolled participants, 69 (82%) completed all treatment courses and ECG measurements. In the primary analysis, the mean increase in QTcF was 17.1ms (SD 17.1) in the third course post dosing ECG compared with 19.6ms (SD 17.8) in the first course post dosing ECG (risk difference -2.4 [95%CI – 6.9 to 2.1], p-value=0.285). We recorded QTcF prolongation >60 ms from baseline in 2 (2.9%) participants after the third course, compared to 3 (4.3%, p-value=1.00) after the first course. None of the participants had QTcF intervals >500ms. Three repeated courses of DHA/PQP given monthly are as safe as single course. DHA/PQP could be administered in consecutive monthly courses as part of elimination strategies, with no increased or cumulative risks in comparison to single course.
THEMES: Drug-based Strategies