ASTMH 2018, Fitsum G. Tadesse: “Clustering of subpatent Plasmodium falciparum and Plasmodium vivax infections around patients in a low-endemic setting aiming for elimination: Batu Degaga kebelle, Adama Woreda, Oromia, Ethiopia”
Collaborator(s): Armauer Hansen Research Institute (AHRI), Ethiopia
Countries: Ethiopia
Published: 29/10/2018
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 67th ASTMH annual meeting in New Orleans, October 2018
Title: “Clustering of subpatent Plasmodium falciparum and Plasmodium vivax infections around patients in a low-endemic setting aiming for elimination: Batu Degaga kebelle, Adama Woreda, Oromia, Ethiopia“
Speaker: Fitsum G. Tadesse, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Session information:
October 29, 2018, 10:15 AM – 12:00 PM, Marriott – Sheraton – Rodrigue Gallery (1st Floor)
Abstract:
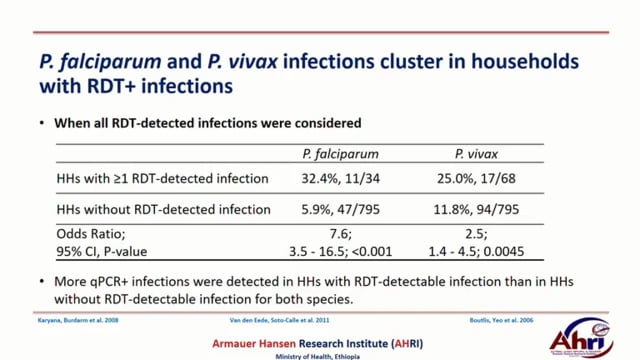
The heterogeneous distribution of infections in settings aiming for malaria elimination challenges finding and treating all relevant infections. In this study we carried out a reactive active case detection: family members and immediate six neighbors of index cases (patients with RDT-confirmed malaria) and controls (who visited the health facility for non-malaria cases) for malaria using RDT and quantitative PCR between October and December 2016 in Batu Degaga kebelle, Adama Woreda, Oromia, Ethiopia. Overall parasite prevalence was 9.7%(90/931) by RDT and 24.0%(218/907) by qPCR. qPCR-detected P. falciparum (Pf) infection prevalence was higher in the community surrounding index cases (13.9%) compared to controls(9.5%; P=.038) while there was no difference for P. vivax (Pv) (P=.926). Four geographically non-overlapping hotspots of any malaria cases with relative risk of 2.11, 1.90, 1.89 and 1.86 were detected (within 280-590meters radius) using SaTScan. Self-reported previous malaria episodes were higher in individuals who lived within risk areas compared to outside risk areas (33.1%, 177/233 vs 1.5%, 3/203; odds ratio[OR], 32.9; 95% CI, 10.2–106.3). Family members who live in households with ≥1 RDT-confirmed Pf individual were considerably more likely to have qPCR-detected Pf infection (32.4%, 11/34) compared to individuals in households without RDT-detected infection (5.9%, 47/795; OR, 7.6; 95% CI, 3.5–16.5; P<.001). Similar findings were found for Pv; (25.0%, 17/68) vs (11.8%, 94/795; OR, 2.5; 95% CI, 1.4–4.5; P<.0045), respectively. Outdoor activities (staying outside late in the evening and leaving early morning) and presence of eave opening were found associated with malaria risk. Data on relatedness of infections within and between households is being assessed using microsatellite markers and will be completed in the coming months. The findings in the present study indicate that subpatent infections are clustered around patients that present to health facilities and detected by standard diagnostics. This suggests that interventions targeted to index case households may support malaria elimination initiatives.
THEMES: Asymptomatic Reservoir | P. vivax