ASTMH 2017, Angana Mukherjee: “A systemic functional analysis of the phosphoinositide metabolic pathway in Plasmodium falciparum”
Collaborator(s): Laval University (UL), Canada
Published: 08/11/2017
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 66th ASTMH annual meeting in Baltimore, November 2017
Title: “A systemic functional analysis of the phosphoinositide metabolic pathway in Plasmodium falciparum“
Speaker: Angana Mukherjee, Laval University, Canada
Session information:
Symposium 0119: “Malaria: Advances in Modeling and Technology for Malaria”
Wednesday, 8 November, 8:00 – 9:45 AM, Convention Center – Ballroom I (Level 400)
Abstract:
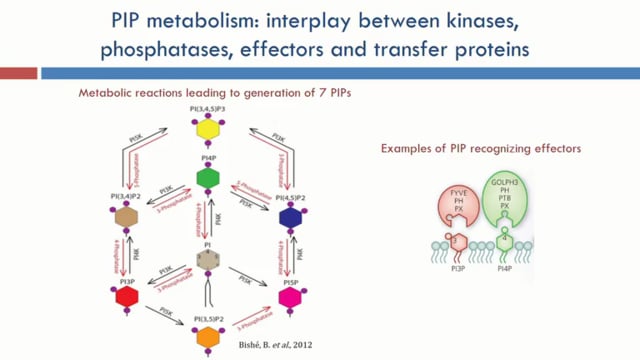
Phosphoinositides (PIPs) are critical components of cellular membranes in eukaryotes, playing massive roles in signal transduction, cell motility, cytoskeletal reorganisation, DNA synthesis, cell cycle, adhesion, membrane transport, permeability and intracellular trafficking. Collectively, seven isoforms of PIPs, the protein effectors that bind them and enzymes that generate or modify them compose a remarkably complex protein-lipid signalling network. Despite their crucial role in eukaryotes little is known about PIP metabolism and functions in the malaria parasite Plasmodium falciparum where the PIP profile is much complex than in uninfected RBCs. Here, in this study we performed a comprehensive knock out screen, by employing the recently reported Selection-Linked Integration (SLI) method to select for targeted gene disruptions (TGD), wherein we attempted to disrupt the complement of the PIP metabolic pathway including PIP kinases, phosphatases, putative PIP effectors and PIP transfer proteins, a total of ~30 targets to identify the genes that are essential for asexual proliferation in P. falciparum. Integration of the SLI-TGD vector at the correct genomic locus was monitored by PCR products across the 5’ and 3’ integration junctions and the quantitative absence of the original WT locus. Our study shows that >50% of the genes in the PIP metabolic pathway could not be disrupted and are likely to be essential for the maintenance of parasite viability, which correlated well with the sparse essentiality data from homologs in P. berghei or T. gondii. This lack of redundancy points not only to the vital role of a number of individual kinases/effectors/phosphatases but also to a potentially abundant source of anti-malarial targets. We will describe asexual growth, susceptibility to antimalarials and gametocytogenesis in the knock outs generated. Additionally, we have also employed the recently reported strategy of knock sideways to localize native proteins by fusing with GFP and functionally analyzing the essential PIP kinases at the protein levels by mislocalizing the native proteins.
THEMES: Enabling Technologies & Assays