ASTMH 2016, Michael F. Good: “Development of a semi-synthetic whole parasite vaccine”
Collaborator(s): Institute for Glycomics, Griffith University, Australia
Published: 14/11/2016
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 65th ASTMH annual meeting in Atlanta, November 2016.
Title: “Development of a semi-synthetic whole parasite vaccine”
Speaker: Michael F. Good, Institute for Glycomics, Griffith University, Australia
Session information: Scientific Session 34: Malaria: Vaccines – Diverse Approaches
Monday, 14 November, 1:45 – 3:30pm, Marriott – Marquis C
Abstract:
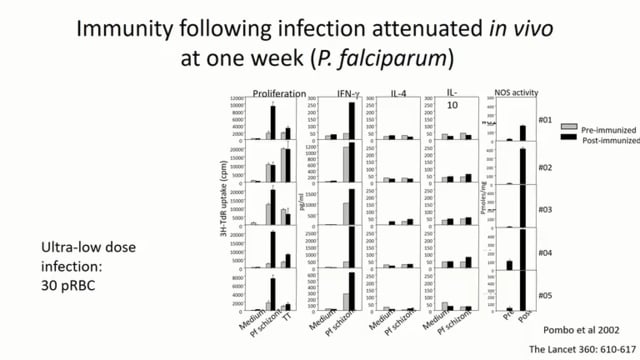
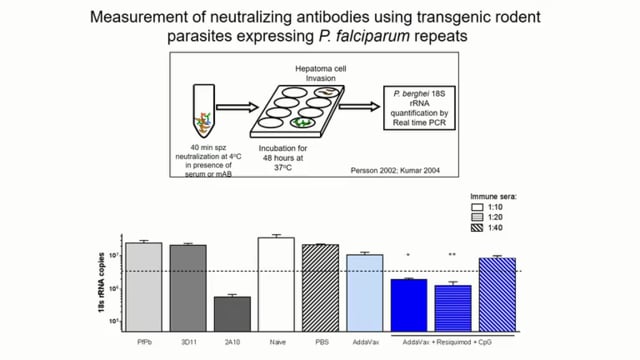
We are developing whole parasite vaccines to protect against the blood stages of malaria and showed that chemically attenuated vaccines can protect against rodent malaria parasites. This approach is being translated to the clinic. However, issues relating to production, storage and delivery present obstacles that impede development of this vaccine and other whole parasite approaches. We are therefore developing a synthetic vaccine delivery system in which killed blood stage parasites are encapsulated within liposomes that are targeted to dendritic cells using mannosylated lipid core peptides (MLCPs). MLCP-liposomes were taken-up efficiently by antigen presenting cells which then upregulated expression of MHC-ll and co-stimulatory molecules, CD80 and CD86. Immunization of mice with MLCP-liposome vaccine formulations, without adjuvant, generated enhanced levels of activated T cells in peripheral blood and vaccinated mice were completely protected from challenge infection with different species of rodent malaria. Liposome formulations are highly promising delivery systems for a human malaria vaccine.