Keystone Symposia “Malaria: Confronting Challenges from Drug Discovery to Treatment” – 2022: Day 3
Wednesday, 13th April 2022
Published: 19/04/2022
This report is brought to you by the MESA Correspondents Patricia Doumbe Belisse, Nutpakal Ketprasit, and Samuel Blankson. Senior editorial support has been facilitated by the Chairs and Speakers of the session.
THEMES: THEMES: Basic Science | Epidemiology | Health Systems
MESA Correspondents bring you cutting-edge coverage from the Keystone Symposia 2022 “Malaria: Confronting Challenges from Drug Discovery to Treatment”
Day 3: Wednesday, 13th April 2022
Session 5 – Reducing the Burden of Malaria Part I
Thierry T. Diagana (Novartis Institute for Tropical Diseases, USA) started by presenting the wide range of Novartis drug candidates for malaria elimination (lumefantrine, KAF156 (ganaplacide), KAE609, INE963 among others). Their three pillars are to meet current demands, address anticipated future needs and early access to drugs for a malaria free world. They are acting in the second major era (2020-2030) to improve existing therapies and address the threat of drug resistance. NITD aims to deliver novel combinations therapies for severe and uncomplicated malaria, chemoprevention, single-dose MDA and P. vivax radical cure to target numerous unmet needs for control and elimination. Diagana pointed out one of the past challenges of poor understanding of hypnozoite’s biology which hindered progress of radical cure drug discovery, however with tremendous efforts over the past decade now we have robust in vitro culture systems, animal models and transcriptomic data to understand the biology. Their ongoing experiments are focused on phenotypic screening. Diagana reported promising results from a Phase IIb study for novel non-artemisinin-based combination therapy to treat uncomplicated malaria: Ganaplacide/lumefantrine combination was efficacious in children less than 12 years old. Diagana also mentioned profile QSAR (PQSAR), which is a machine learning-based method for virtual screening of drugs. Using an artificial intelligence approach, they can distinguish three distinct mechanisms of action (MOA) by phenotype.
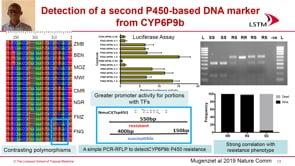
Abdoulaye Djimde (University of science, Techniques and Technologies, Mali) presented genomics studies as a novel tool to reduce the burden of malaria in Africa. Djimde spoke about the missions of Plasmodium Diversity Network Africa (PDNA) to build capacities of African scientists and use knowledge from malaria parasite genetic diversity to inform malaria elimination policies in sub-Saharan Africa. Among other things, PDNA helps with regular monitoring of molecular markers involved in parasite drug resistance (PfK13). A report many years ago showed that Southeast Asian parasites were diverse whereas African parasites were closely related in a PCA analyses. However, diving deeper into the data shows that in fact there is high genetic diversity of P. falciparum African parasites. Keeping this parasite diversity in mind, accordingly there should be a diversification of treatment strategies depending on each locale. Djimde presented malaria sub-national stratification in Mali. The complexity and significant differences in the risk of malaria infection in different locations of southern Mali suggests the need of district tailored interventions. He also highlighted the use of genetic epidemiology by the national malaria control program in the region for control and elimination of malaria. In addition, Djimde mentioned that parasites with HRP2/3 deletion will lead to a false negative test using rapid diagnostic tests (RDT), which is widely used. Many places do not have a constant supply of electricity, microscopists, or microscopes, so routine diagnosis by microscopy cannot be achieved. Other cheap diagnostic tools need to be invented and distributed. Djimde ended his communication by highlighting the need to translate genomics knowledge into policy decisions.
Janice Culpepper (Bill and Melinda Gates Foundation, USA) started her talk by informing about the shift in the Foundation’s malaria elimination strategy (2019 onward) now referred to as the “pathway to eradication” strategy. The Bill and Melinda Gates Foundation’s strategy covers targeting high burden areas referred as “Eradication backbone”, optimizing the use of existing tools and supporting the development of new tools required for elimination. Culpepper further illustrated the benefit of single exposure radical cure (SERC) which not only includes effectiveness, but also simplifies case management, and improves compliance. The next generation of drug combinations will be focused on delivering a SERC. Currently, ivermectin is used as an endectocide to reduce and eliminate residual transmission. However, modeling suggests that a short-course ivermectin is unlikely to be an ideal elimination tool. Therefore, long-acting endectocides are needed to reduce and eliminate residual transmission, while mass drug administration will extend the duration of parasite reduction in the population. Culpepper also highlighted the importance of Chemoprevention. Culpepper mentioned that new strategies including novel and repurposed drugs, long-acting injectable drugs for seasonal prevention, and monoclonal antibodies are needed. Besides these existing tools, robust surveillance systems are needed.
Beatriz Baragana (University of Dundee, UK) and her group identified Plasmodium falciparum acetyl-CoA synthetase (PfAcAS), as a target of MMV019721, a multistage antimalarial, by in vitro selection followed by whole genome sequencing; resistant parasites had mutations in PfAcAS that line the predicted active site of the enzyme. This was confirmed by showing that PfAcAs WT parasites genetically edited to express A597V or T648M yielded resistance to MMV019721 similar to that seen in the selected resistant lines. In addition, conditional knockdown of PfAcAS sensitized parasites to MMV019721, demonstrating that this protein is necessary for resistance to the compound. Using recombinant WT or mutant PfAcAS, they found linear non-competitive inhibition for the compound vs ATP, and linear competitive inhibition against CoA. Thus, MMV019721 inhibits CoA from binding to the enzyme, resulting in the inability of PfAcAS to catalyze formation of acetyl coA. They were able to reproduce co-crystallisation with the MMV-019721 analogue using ethyl-AMP and are still working on obtaining a full length Cryo EM PfAcAS structure. Meanwhile, they have started working on MMV-019721 optimization to increase the potency, solubility and metabolic stability without the structural information and the outcome suggests that this compound is ready to enter lead optimization (pharmacokinetics and potency studies).
Lauren B. Arendse (University of Cape Town, South Africa) shared her current work on P. falciparum kinases as antimalarial targets. Set of 84 human kinase inhibitors were screened against asexual blood stage parasite and kinobead study. Also, her team and colleagues used chemogenomics to explore more potential kinase inhibitors. ADP-Glo assay was used for validation. One compound showed polypharmacology and inhibited two P. falciparumkinases: PfPI4K and PfPKG. These kinases were found essential across the parasite life cycle, and therefore suitable for antimalarial targets. For instance, MMV030084 was found to inhibit liver stage, asexual blood stage, and male gametocyte of P. falciparum. Arendse proposed that polypharmacology, where several plasmodium kinases are inhibited by the same inhibitor, could be a potential opportunity in antimalarial development.
Christian Doerig (RMIT University, Australia) presented a new aspect of anti-infectives. Prof. Doerig and his team focuses on host-directed therapy for malaria, because this approach will reduce the ability of parasites to mutate to acquire drug resistance. In this approach, treatment of infectious diseases is focused on host cell factors required for parasites. Doerig’s team employed a Kinexus antibody microarray for exploring host-cell phospho signalling responses to infections. This method has been validated in many infectious agents including Hepatitis C. His team then applied the same method to study the signalling response in malaria-infected erythrocytes, leading to identification of human MEK1, c-MET and B-Raf that are targeted by inhibitors. They were unable to raise resistant lines from such inhibitors, suggesting refractoriness to resistance, which is a desirable trait in antimalarial drugs. The future work is to elucidate the mechanism of actions of the characterized inhibitors.
Stuart A. Ralph (The University of Melbourne, Australia) presented work on apicoplast inhibitors and decreased hemoglobin digestion. Apicoplast inhibitors such as the antibiotics clindamycin, doxycycline, indolmycin, etc. cause delayed death in parasites due to isopentenyl pyrophosphate (IPP) deprivation. IPP is used in protein prenylation, which are on proteins that interact with membrane proteins or vesicles. Using 3D Block-face SEM, Ralph’s group found that indolmycin treatment leads to cytostomal invaginations that are abnormally long and winding, and disrupted digestive vacuoles (DV); this effect can be rescued by geranyl geranyl alcohol (GGOH). There is also a decrease in hemoglobin-derived peptides as measured by metabolomics. The working hypothesis is that antibiotics lead to defects in rab-based trafficking of the cytosome to the DV. K13 forms a ring/”collar” around cytostomes. In K13 knock sideways experiments (in which K13 is mislocalized), the cytostome collar is opened up. Treatment with doxycycline or clindamycin results in opening up of the collar similar to when K13 is mislocalized, as seen by microscopy. Hemoglobin metabolism is also similarly impaired when treated with these antibiotics or when K13 is mislocalized, as detected by mass spectrometry studies. Drug interaction analysis revealed antagonistic interaction between dihydroartemisinin and delayed death drugs (clindamycin, and doxycycline). It is suggested that this is because antibiotics reduce hemoglobin uptake due to the inability to traffic from the cytostome to the DV and thus there is lower amount of free heme to activate artemisinin.
Session 6: Reducing the burden of Malaria Part II
Philip Rosenthal (University of California, USA) gave an overview of key antimalarial drugs deployed in Africa for treatment (artemisinin-based combination therapy – ACT) and for prevention (sulfadoxine-pyrimethamine – SP). To look for antimalarial drug resistance, they performed clinical trials of antimalarial drug efficacy, in vitro drug sensitivity of fresh parasite isolates and resistance surveillance. Recent treatment efficacy of ACTs in Uganda highlighted efficacies of artemisinin-lumefantrine (AL), artesunate-amodiaquine (AS/AQ) with an improved efficacy of AS/AQ compared to AL. Ex-vivo drug susceptibility revealed a change in drug sensitivity in Uganda and transporter genotypes. He also reported evidence for artemisinin resistance in Rwanda and Uganda with K13 mutations. Regarding the decreasing resistance to aminoquinolines and increasing resistance to SP, he calls for a continued surveillance of change in drug sensitivity.
Elizabeth A. Ashley (Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit, Laos) talked about the increasing artemisinin resistance in Asia and Africa and the need to move towards new combinations treatments through triple artemisinin-based combinations or TACTs. This approach showed clinical relevance when treating uncomplicated Plasmodium falciparum malaria. It is presumed that a new non-artemisinin containing treatment will be available in 2027 talking about ganaplacide-lumefantrine as many challenges are rising. Among others, reluctance to adopt TACTs by most countries without evidence of efficacy in local context, huge increase in cost of drugs compared to the status quo. Many TACTs are under evaluation and implementation considerations such as safety and tolerability concerns and market positioning need to be addressed. TACTs are an option to integrate with other strategies to delay emergence of resistance to artemisinin or partner drugs in countries where it has not been reported.
Ric Price (Menzies School of Health Research, Australia) talked about novel approaches for the safe and effective radical cure of P. vivax, where the highest burden is found in children and in remote areas. Other challenges to the radical cure of P. vivax malaria include poor sensitivity of parasite diagnostics, variable risk of relapse, safety concerns, poor adherence, and lack of sustained financing. He then identified new tools and strategies for tackling P. vivax malaria including areas such as parasite diagnostics, treatment regimens, G6PD diagnostics, adherence to drug regimen and safety of drugs. Diagnosis of P. vivax requires experienced, trained microscopists and specific rapid diagnostic test (RDTs) kits. Primaquine (PQ) and Tafenoquine (TQ) are the main anti-hypnozoite drugs used for the treatment of P. vivax malaria. The combination of these drugs with/in ACTs show that PQ works better, and that high doses of PQ is a more efficient treatment. He also stated that the commonest cause of treatment failure is underdosage and poor adherence on the part of both patients and prescribers. Some patients don’t complete the 14-day regimen for an acute febrile illness leading to relapse. Thus, malaria surveillance and proper prescriptions are required. Finally, he mentioned G6PD deficiency and its associated haemolytic risk; and listed quantitative G6PD testing aside qualitative G6PD testing as a new alternative though costly.
Marielle Bouyou Akotet (University of Health Science, Gabon) started by stating the mission of Malaria National Control Program (MNCP) which is to provide the best interventions for the prevention and treatment of malaria with the view of eliminating malaria through co-ordinated efforts of several bodies and partners. She presented data on the trend of malaria cases and malaria related deaths nationwide and in Africa. She emphasized that there hasn’t been any change in malaria prevalence and death during the period from 2015 – 2020. She mentioned the goals and some interventions already in place to control malaria such as the distribution of insecticide treated nets (ITNs), indoor residual spraying (IRS), and Sulfadoxine-pyrimethamine (IPTP-SP) coverage in some key malaria transmission sites. She attributed causes to the challenges confronting them to include patient behaviors such as not seeking care and self-medication with anti-malarial drugs, quality of case management, poor diagnosis, little or no data, poor access to remote sites, and involvement of different stakeholders. She then recommended the setting up of sentinel sites for malaria survey.
Lauriane Sollelis (University of Glasgow, UK) started her talk by mentioning that parasite adaptability enables survival and that there is natural selection for variation in sexual conversion rates across parasite populations and time. Sollelis and her team sought to find the genetic determinants that select for parasite transmission, and which may facilitate the spread of drug resistance in Plasmodium falciparum. Using a genome wide association study, they measured the expression of an essential gametocytogenesis gene, ap2-g, across tracking resistance to artemisinin collaboration (TRAC-1) samples. Their studies found two lncRNAs, Ben and Glen, that are genomic determinants of stage conversion in P. falciparum; and that the geographical distribution of high/low ap2-g expression alleles are associated with low and high transmission areas. Lastly, she showed that the spread of artemisinin resistance is associated with the presence of high ap2-g allele expression.
Meeting Wrap-up: Outcomes and Future Directions
After the last talk of session 6, the Chairman, Collin Sutherland handed over to Elizabeth Ashley, one of the organizers of the symposium. She gave the vote of thanks and thanked everyone including the organizers, attendees both in person and virtual, the various enlightened speakers and poster presenters who shared their work and insights, as well as the sponsors of the event. David A. Fidock gave the closing remarks but not without appreciating his fellow organizers and the Keystone Organization who played a key role in the event. Finally, he asked attendants to fill the online survey as the feedback from the survey would help in decision making and policies for future events.
This report is brought to you by the MESA Correspondents Patricia Doumbe Belisse, Nutpakal Ketprasit and Samuel Blankson. Senior editorial support has been facilitated by the Chairs and Speakers of the session.
Published: 19/04/2022
This report is brought to you by the MESA Correspondents Patricia Doumbe Belisse, Nutpakal Ketprasit, and Samuel Blankson. Senior editorial support has been facilitated by the Chairs and Speakers of the session.
THEMES: Basic Science | Epidemiology | Health Systems