6th International Conference on Plasmodium vivax Research (ICPvR) – 2017: Day 2
Monday, 12th June 2017
Published: 10/12/2018
This report is brought to you by the MESA Correspondents Rosalind Howes, and Hernando del Portillo.
THEMES: THEMES: Genetics and Genomics | P. vivax
MESA Correspondents bring you cutting-edge coverage from the 6th International Conference on Plasmodium vivax Research
Day 2: Monday 12th June
The conference’s main scientific meeting got off to an impressive start with 33 talks covering a wide range of themes, grouped into the following topics.
Topic 1: Tackling the hypnozoite and relapse
This opening session put end to any lingering doubts of hypnozoites remaining “invisible” or “dormant”.
Steven Maher presented his hypnozoite liver model, a primary hepatocyte liver-stage culture system which allows not only morphological characterisation of schizonts and hypnozoites but also candidate drug screening. A hypnozoite liver model is also being used by Alison Roth to screen pre-erythrocytic interventions and identify an antibody as a marker of liver infection.
Humanized mouse liver systems (>90% human hepatocytes) developed by Sebastian Mikolajczak are providing insight into hypnozoite maturation patterns, and visualisation of the impact of primaquine under optimised dosing regimens.
Impressive laser-capture microdissection is allowing Roger Cubi and Shruthi Vembar to isolate individual schizonts and hypnozoites of liver parasites in P. cynomolgi for single-cell transcriptomics. Similar transcriptomic evidence of hypnozoite non-dormancy in P. cynomolgi was presented by Anne Marie Zeeman. Further evidence of hypnozoite activity from detection of hypnozoite-derived exosomes in plasma was proposed by Melisa Gualdrón-López as an easily accessible biomarker of hypnozoite infection identifiable from a panel of protein markers.
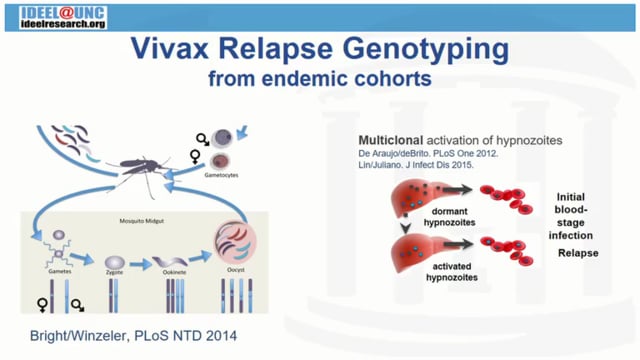
Different immunological responses between primary infection and relapses presented by Chet Joyner showed the role of strain-specific B-cell memory; while deep sequencing of pvmsp1from successive Pv relapses indicate high multiplicity of infection in Cambodia, with differentiated waves of clonal activation through time, as discussed by Jessica Lin.
Finally, Dennis Shanks proposed a hypothesis grounded on historical military data of haemolysis as a trigger of relapse, with implications for the therapeutic mechanism of primaquine.
Topic 2: Achieving universal access to safe and effective radical cure
Lucio Luzzatto opened this next session with a historical overview of the “malaria-primaquine-G6PD deficiency” triangle. He highlighted common misconceptions around G6PD deficiency, including G6PDd being more frequent in males (in reality heterozygote females are roughly twice as frequent) and G6PDd being recessive. He discussed the need for quantitative phenotypic assessment of G6PD activity in females to predict haemolytic risks from primaquine as genotyping only conveys an incomplete characterisation. Kevin Baird developed this theme with discussion of the factors causing the “erosion of primaquine effectiveness in the real world” which represent important programmatic barriers to achieving safe radical cure of the hypnozoite reservoir. The heavy clinical burden of relapse, however, demands practical solutions to this treatment dilemma.
These overviews paved the way for much-anticipated presentations on the results of the GSK/MMV-driven Tafenoquine trials: DETECTIVE and GATHER, presented by Raul Chuquiyauri and Marcus Lacerda, respectively. The DETECTIVE phase III clinical trial demonstrated the efficacy of single-dose Tafenoquine (70% reduction in recurrence at the 6 month end-point relative to placebo), with GATHER demonstrating a good safety profile and no evidence of drug-induced adverse events. A limitation of GATHER was the low recruitment of heterozygous G6PD deficient P. vivax-infected females, something subsequently addressed with primaquine by Cindy Chu. Her study assessed risk of primaquine-induced haemolysis in phenotypically normal G6PDd heterozygote females, finding that the standard dose (0.5mg base/kg daily for 14d) was well tolerated in all phenotypically normal G6PDd female participants.
Annette Erhart’s work in Vietnam corroborated earlier findings of P. falciparum being a high risk factor for subsequent P. vivax relapse, suggesting a benefit for more widespread use of primaquine.
Rob Commons’ pooled analysis of the haematological profile of chloroquine and primaquine treatment identified differential Hb trends related to baseline Hb levels. Patients with lower baseline Hb levels (< 10g/dL) showed only an increase in Hb following treatment, contrasting with an initial marked drop seen when baseline levels higher.
Finally, a call was made by Chansuda Wongsrichanalai for simple and programmatically relevant case management algorithms for primaquine therapy, something currently very variable between countries.
Topic 3: Novel insights into parasite biology
This session was principally focussed on parasite invasion pathways, with Wai-Hong Tham presenting the structure of the PvRBP2 ligand which binds to reticulocyte transferrin receptor CD71 to achieve cell invasion. Blocking antibodies (anti-PvRBP2b) demonstrated to inhibit P. vivax invasion, varied in efficacy between samples, reminding us of the “everlasting host-pathogen arms race”. Benoit Malleret followed this up with his announcement of identifying CD98, an erythrocytic amino acid transporter, as another important P. vivax invasion receptor, binding PvRBP2a in a dimer-dimer conformation. Selective inhibition of these receptors could be developed as an invasion-blocking intervention. Usheer Kanjee discussed methods using quantitative surface proteomics to identify potential new receptors, confirmed the well-known role of DARC, and demonstrated the use of knock-out technologies to down-regulate expression of TfR receptor to significantly reduce invasion.
Adeline Chua discussed her success with achieving up to 10% parasitaemia of P. cynomolgi in continuous culture, a valuable model for P. vivax drug screening.
Several speakers, including Nicanor Obaldia, then spoke to the role of bone marrow in harbouring P. vivax parasitaemia. Marcelo Brito identified 274 P. vivax genes from the bone marrow transcriptional profile with changing gene expression over time directly linked to erythropoiesis and potentially indicating an association with P. vivax anaemia.
Luis Carlos Salazar-Alvarez presented evidence of increased P. vivax gametocyte rosetting being associated with increased infectivity to Anopheles aquasalis vectors, and Wanlapa Roobsoong summarised results from Ethiopia relating PvDBP copy number with asymptomatic infection.
Topic 4: Systems biology
The day’s final session featured studies using “big data” approaches for drug screening and parasite biology elucidation.
Elizabeth Winzeler’s work aims to identify a universal marker of infection from the complex human transcriptome that is “switched on” by infection; results indicate that hsMUC13 may act as a universal biomarker for human malaria infection, though it is uncertain whether this responds to hypnozoites. Mark Styczynski presented data from a P. cynomolgi model in rhesus macaques that generates extensive “systems biology” datasets at repeated timepoints during primary and relapse infections (with relapses showing markedly lower upregulation), including proteomics, genomics, lipidomics and metabolomics. He discussed solutions to “integrating the omics” in situations when expected correlations do not emerge; these complex relationships provide added insight into the underlying biology. Luiz Gardinassi investigated differential metabolic signatures to clinical outcomes through controlled human P. vivax infections of naïve and semi-immune hosts, finding that these varied between host groups at all time points during infection. Karan Uppal demonstrated the role of metabolomics in identifying biomarkers of chloroquine-resistant P. vivax malaria and an integrative network analysis combining the metabolomics with clinical outcomes.
Rosa Santana presented complimentary results from transcriptomes of P. vivax-infected Anopheles darlingi and An. aquasalis mosquitoes, demonstrating differential gene expression during invasion of the midgut epithelium by the two vector species. Alison Roth also applied transcriptome analysis in the search for infectivity genes in P. vivax sporozoites in the study of the parasite’s transition phases from vector into host.
Sonia Agrawal presented a method based on a whole genome capture technique for improved quality of P. vivax genome sequencing for samples with high levels of human DNA contamination.
“SPOTmalaria” was presented by Cristina Ariani, a collaborative MalariaGEN-led effort for genomic surveillance of the global evolution of malaria parasites. This open-access population genomics database uses state-of-the-art technologies to simplify and accelerate genetic data reporting to allow rapid identification of resistance emergence and other trends in parasite populations.
This blog was written by Rosalind Howes (University of Oxford) and Hernando del Portillo (Barcelona Institute for Global Health (ISGlobal) & Institut d’Investigació Germans Trias i Pujol (IGTP)) as part of the MESA Correspondent program, and is cross-posted on the MESA website and Malaria World.
Published: 10/12/2018
This report is brought to you by the MESA Correspondents Rosalind Howes, and Hernando del Portillo.
THEMES: Genetics and Genomics | P. vivax