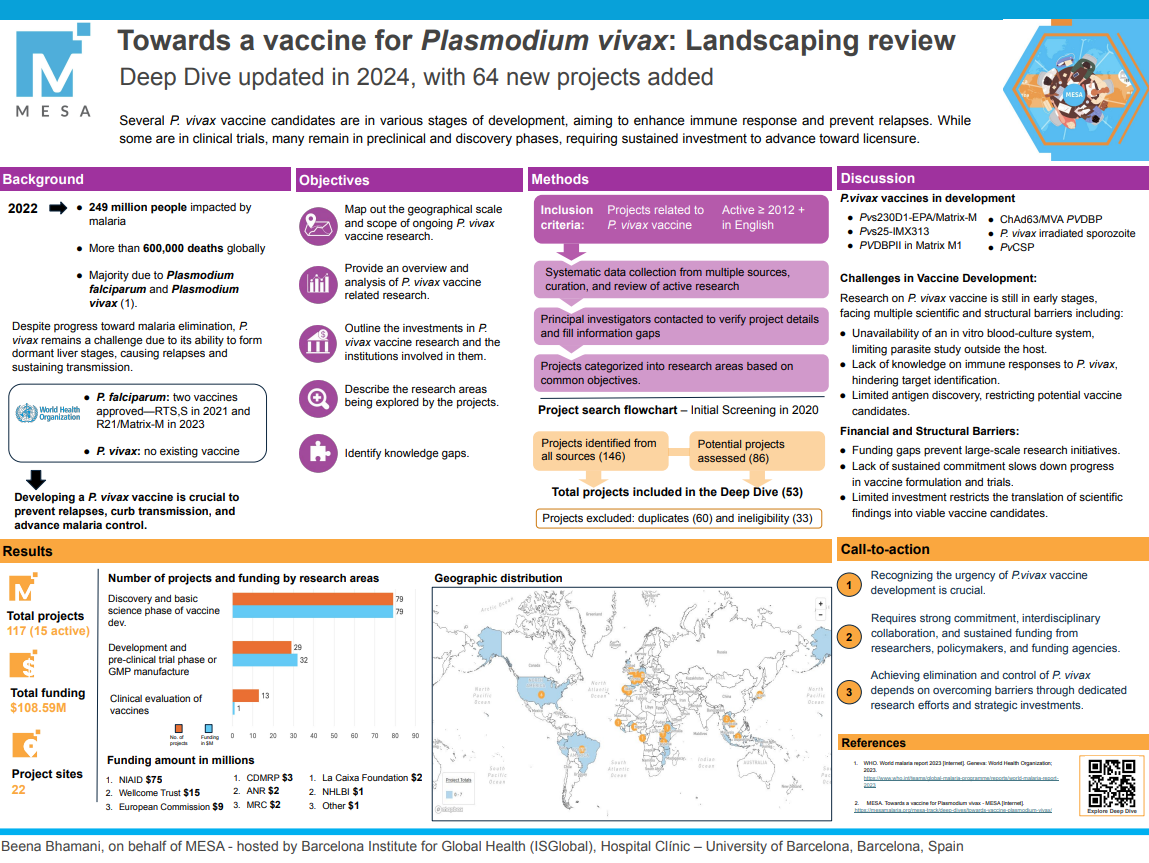
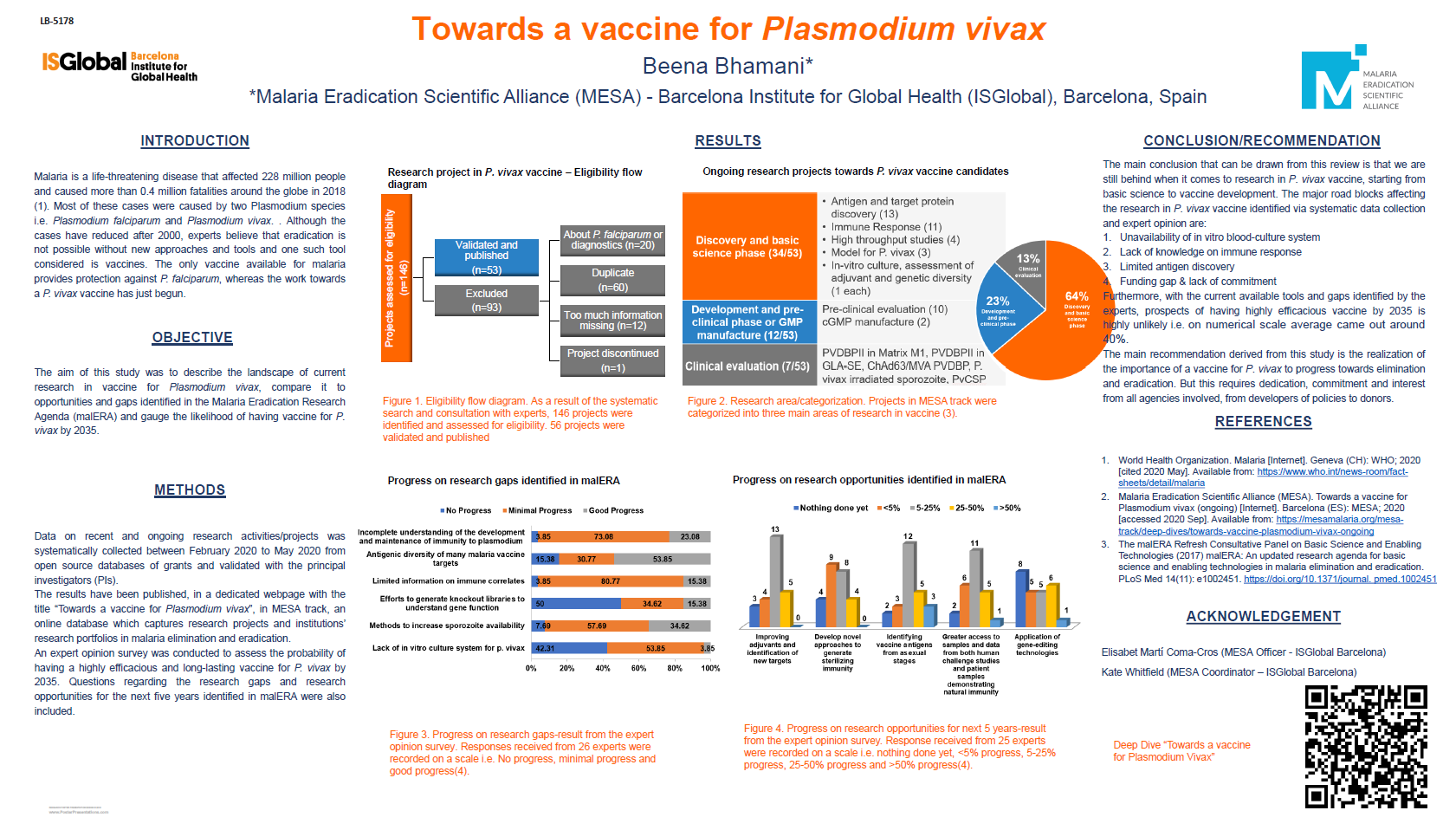
8th Virtual International Conference on Plasmodium vivax Research (ICPvR) – 2022: Day 2
Wednesday, 6th April 2022
Published: 07/04/2022
This report is brought to you by the MESA Correspondents Melina Florez-Cuadros, Varun Gorki, Neha Sylvia Walter, and Duru Vincent Chiagozie. Senior editorial support has been facilitated by the Organizers and Chairs of the sessions.
THEMES: THEMES: Basic Science | Epidemiology | Health Systems | P. vivax
MESA Correspondents bring you cutting-edge coverage from the virtual 8th International Conference on Plasmodium vivax Research (ICPvR 2022)
Day 2: Wednesday, 6th April 2022
Keynote presentation
Luzia H. Carvalho (Oswaldo Cruz Foundation, Brazil) initiated her keynote address by giving an overview of what is generally known about the antibody response to the DBPII (Duffy Binding Protein II) and highlighted its genetic variability in endemic areas, which is the major ligand for P. vivax invasion of erythrocytes, and also highlighted the potentials of this ligand holds as a vaccine candidate, given its ability to elicit IgG antibodies that are able to block the interaction of DBPII with the Duffy Antigen Receptor for Chemokines (DARC). However, only a low proportion of P. vivax exposed individuals actually acquire the strain-transcending blocking inhibitory antibodies (BIAbs) response which she called “the elite responders”. The DBPII ‘elite responders’ were found to be unrestricted to areas of high transmission alone considering their presence in the Brazilian Amazon areas. Also, using a synthetic DBP immunogen (DEKnull2), associated with strong and persistent naturally-acquired IgG antibody followed by IgM antibodies response to provide a strong and persistent response, suggested promising results. However, there were differences in the gene expression (Allele a/Allele b) in elite responders versus the non-responders. Altogether, these results are encouraging and elicits a potent BIAb response.
Session 3: Invasion and biology of vivax malaria
Wanlapa Roobsoong (Mahidol Vivax Research Unit, Thailand) X-rayed the various experimental models of P. vivax culture that have been successfully used in studying the liver stages in their lab. For the in vivo models, two humanized mouse models support complete liver stage development and hypnozoite formation. The HC-04 cell line allows in vitro schizont and hypnozoite culturing. This cell line also supports in vitro reactivation of hypnozoites and genetic manipulation of host hepatocytes.
Considering the large variations of P. vivax field isolates, Gigliola Zanghi (Center for Global Infectious Disease Research, United States), in collaboration with other groups, developed a Chesson strain liver stage model employing the sporozoites (PvSPZ) of the Chesson strain. Chesson blood stages parasites were propagated in Saimiri monkey and fed to the mosquitoes for PvSPZ production and cryopreservation. The PvSPZ was inoculated in human liver-chimeric FRG huHep mice and the liver-stage parasites were analysed by single cell RNA-seq. The group observed the exo-erythrocytic schizonts formation over nine days and a typical ratio of 40% hypnozoites: 60% schizonts in the infected livers. The Chesson strain liver stage model will enable the identification of innovative therapies to prevent relapse by providing a better knowledge of hypnozoite biology.
Anthony Ruberto (Center for Tropical and Emerging Global Diseases, USA) presented a study elucidating the molecular signatures of P. vivax hypnozoites and human hepatocytes using single-cell RNA sequencing. On the parasite side, the study revealed distinct transcriptional signatures between the hepatic schizont and the hypnozoite stage. On the human side, Ruberto highlighted the transcriptional responses of hepatocytes infected with hypnozoites or schizonts and observed that the pathway associated with immune responses was down-regulated while the energy metabolism genes were enriched. This study offered insights into P.vivax liver-stage biology and revealed host and parasite markers that may be targeted for new antimalarials development.
Prasun Kundu (Cambridge Institute for Medical Research, UK) reported a study showing the role of the Plasmodium Tryptophan Rich Antigens (TRAgs) in blood stages invasion. They employed the HEK293E cell system to express 25 full-length TRAgs of P. vivax. Polyclonal antibodies were raised and used to localize the proteins. Despite the fact that only half of the P. vivax TRAgs were predicted to have an N-terminal signal peptide, immunofluorescence experiments revealed surface staining on merozoites in the P. knowlesi model, regardless of signal peptide prediction. Flow cytometry assays, lipid dot blots, liposome binding experiments and transgenic P. knowlesi (through deletion of the orthologous gene) were used to confirm TRAg25 lipid binding and its role in the invasion of reticulocytes.
Session 4: Towards vaccine development
Mimi Hou (University of Oxford, UK) presented research work on testing the clinical efficacy of the PvDBPII malaria vaccine in a controlled human malaria infection (CHMI) study undertaken in the UK. Two formulations of PvDBPII vaccine were developed: a viral vectored and a protein in adjuvant (Matrix-MTM). Study findings indicated better immunogenicity and efficacy of a fortuitously delayed dosing regimen of the protein-in adjuvant than the viral vectored vaccines. A 51% reduction in the in vivo parasite multiplication rate, and corresponding high antibody titres good effects were observed in this group compared to the control and viral vectored groups. They also established an in vivo growth inhibition activity (GIA) assay using P. knowlesi transgenic for PvDBP and showed a correlation with vaccine efficacy using this assay, pointing the way to a way of predicting vaccine efficacy in the future.
Ganesh Ram Visweswaran (Seattle Children’s Research Institute, United States) called attention to the matter that secondary infections caused by hypnozoites relapsed in P. vivax can account for 90% of clinical cases, which also impact transmission. They reported the use of the FRG humanized mouse liver model to assess the ability of CSP antibodies to arrest schizont development and hypnozoite formation. The experiments showed that mice infused with CSP antibodies had reduced relapses when the antibody was administered at a suboptimal dose.
Sumana Chakravarty (SANARIA, United States) and colleagues reported the development of a resource to provide live Plasmodium vivax sporozoites, akin to the P. falciparum product (PfSPZ). The SANARIA group aimed to generate stocks of aseptic, purified, vialed cryopreserved PvSPZ that meet the regulatory criteria for human use. They reported the successful production of PvSPZ using a Saimiri boliviensis primate model to produce patent infection with P. vivax gametocytes, then used the infected blood to transmit to Anopheles stephensi mosquitos before harvesting viable sporozoites from the salivary glands of the mosquitos. This resource promises the possibility of a continuous supply of P. vivax sporozoites.
Caroline Junqueira (Oswaldo Cruz Foundation, Brazil) and her group used immunopeptidomics analysis on human leukocyte antigen (HLA) class I molecules expressed on the surface of P. vivax-infected erythrocytes to identify more than 450 peptides that belong to 167 parasite proteins, from samples of seven patients. These proteins included housekeeping proteins, proteins conserved across species and common among the patients studied. After assessing the immune response to these peptides and also, MHC restriction analyses, they conclude that P. vivax infected reticulocytes present peptides on both classical and non-classical MHC-I molecules. As a result, P. vivax infected reticulocytes are recognized and killed by the CD8+ T cells. In addition, the study group hypothesized that since most of these proteins are housekeeping proteins, they will likely be presented by infected hepatocytes, too.
Rapid Fire Presentation – Invasion and biology of vivax malaria
#200 Katlijn De Meulenaere (Institute of Tropical Medicine Antwerp – ITM Antwerp, Belgium) stated how the identification of new receptor-ligand pairs for the invasion process of P. vivax is very crucial to uncovering vaccine candidates targeting the parasite erythrocyte stage. They identified a new band3-mediated pathway in P. vivax, with pvTRAg genes being the best ligand candidate. They also showed that linking invasion phenotypes with transcriptomes from the same parasite can inform host-parasite interactions involved in invasion.
#201 Caitlin Bourke (Walter and Eliza Hall Institute - WEHI, Australia) elucidated how the researchers performed a full-length RNA sequencing on an mRNA molecule, inclusive of the 3’ and 5’ untranslated regions (UTRs) that flank the coding sequence, in order to better understand and interpret the P. vivax transcriptome from a sporozoite point of view. The study cataloged isoform variations present in the sporozoites and provided crucial insights into the P. vivax’s sporozoite transcriptional landscape.
#203 By using the cell-line which overexpressed the EphA2 against P. vivax, Sittinont Chainarin (Mahidol Vivax Research Unit, Thailand) strengthened the concept of EphA2 as a hepatocytic receptor for P. vivax infection and provided a platform for more research into the biology of exo-erythrocytic P. vivax-host interactions.
#204 Susanne W. Warrenfeltz (Center for Tropical and Global Diseases, United States) on behalf of the VEuPathDB Team elaborated on, and encouraged researchers to access PlasmoDB and VectorBase databases. They are totally free, easy to use and have the information of genomic-scale datasets and data mining tools of 112 organisms including Plasmodium species and their vectors.
Rapid Fire Presentation – Towards vaccine development
#206 Herbert Opi (Burnet Institute for Medical Research and Public Health, Australia) shared that his group has developed novel luminex assays for quantifying functional immunity in P. vivax infections that helped in assessing immune responses to multiple antigens simultaneously. They were able to identify known and novel antibody targets and functions, useful for vaccine development.
#207 Utpalendu Paul (Vellore Institute of Technology, India) et al. used reverse vaccinology to find highly antigenic P. vivax proteins specifically of Sal1 species and analyzed clinically extracted P. vivax protein using immunoassays. Four proteins (MSPI, MSP8 putative, MSP10 putative and Asparagine-rich protein) were identified as highly antigenic and are potential vaccine candidates.
#208 Sataporn Thongpoon (Mahidol University, Thailand) and the group identified and characterized transmission-blocking vaccine candidates from plasma of patients naturally infected with P. vivax. In addition to an already known vaccine candidate Pvs230, new transmission-blocking vaccine candidates were identified.
#209 Sheena Dass (Harvard T.H. Chan School of Public Health, United States) et al. selected eight antigen P. vivax proteins considering their functional roles in erythrocytes invasion and relatedness with P. cynomolgi and P. knowlesi. Four antigenic antibodies displayed >50% inhibition in both sister species of which high priority antigen Pv_P108 showed the highest level of invasion inhibition in all three species. Thus the proposed model of using P. cynomolgi and P. knowlesi can facilitate identifying vaccine candidates for P. vivax.
#210 Luna de Lacerda (Fiocruz, Brazil) presented her study that evaluated immune response of 50 P. vivax peptides using P. yoelii mice modelsL3 and S2 were the most relevant Pv peptides that induced immune response to Py infection and decreased parasitemia, thus can be potential vaccine candidates
#211 Thomas Martinson (Oregon Health & Science University, United States) along with his group used the P. cynomolgi in Rhesus macaques to assess CD8 T cell killing capacity against iRBCs. They found that CD8 T cell response is dependent on the MHC-E molecule. This validates the human-relevant model for P. vivax studies.
#212 Guilherme Castro (Instituto René Rachou, Brasil) presented his research on the importance of γδT cells in the lysis and phagocytosis of merozoites and infected erythrocytes in patients with P. falciparum as well as and P. vivax malaria.
#213 Cristiana Ferreira Alves de Brito (Institute René Rachou – Oswaldo Cruz Foundation,Brazil) et al. Investigated the IgG antibody titres in neotropical primates naturally exposed to Plasmodium. Their findings indicated high antibody titres in wild adult monkeys than in the captive ones shedding light on the potential reservoirs of malaria infection and the immune response generated in the natural environment.
#214 Herbert Opi (Burnet Institute of Medical Research and Public Health, Australia) and his group assessed the ability of naturally-acquired and vaccine-induced antibodies (Abs) to block the interaction between PvAMA1 and RON2, essential for parasite invasion. The presence of this Abs did not result in protection against clinical malaria but they resulted to be very very cross-reactive, making this interaction an attractive vaccine target.
#215 Iris Aparici Herraiz (Barcelona Institute for Global Health – ISGlobal, Spain) utilized the direct immuno-affinity capture (DIC) technique to identify the CD71+-EVs from the infected reticulocytes of P. vivax malaria patients. They reported DIC as a robust strategy to identify P. vivax immunogenic proteins as antigens for vaccine development.
#216 David Narum (National Institute of Allergy and Infectious Diseases – NIAID, United States) et al. developed and characterized Pvs230D1M and Pvs230D1-EPA which are conjugated to nanoparticles. Both had the ability to block P. vivax transmission using membrane feeding assays with blood from naturally infected individuals from Colombia as well as in Samiri monkeys. They are planning to test the vaccine candidate (cGMP Pvs230D1-EPA with Matrix-MTM adjuvant) in clinical trials.
This report is brought to you by the MESA Correspondents Melina Florez-Cuadros, Varun Gorki, Neha Sylvia Walter and Duru Vincent C. Senior editorial support has been facilitated by the Organizers and Chairs of the sessions.
Published: 07/04/2022
This report is brought to you by the MESA Correspondents Melina Florez-Cuadros, Varun Gorki, Neha Sylvia Walter, and Duru Vincent Chiagozie. Senior editorial support has been facilitated by the Organizers and Chairs of the sessions.
THEMES: Basic Science | Epidemiology | Health Systems | P. vivax