70th ASTMH Virtual Annual Meeting – 2021: Day 4
Saturday, 20th November 2021
Published: 24/11/2021
This report is brought to you by the MESA Correspondents Edima Ottoho, Tope Kayode, Franklin Tembongshu Formilack, Lucy W. Mwangi, Vita Mithi, Ana Alonso, Faith Hungwe, Olajoju Temidayo Soniran, Isabelle Delrieu, Patricia Doumbe Belisse, and Carlos A. Fernández Miñope. Senior editorial support has been facilitated by the Organizers and Co-Chairs of the symposia and Divya Beri.
THEMES: THEMES: Basic Science | Epidemiology | Health Systems
MESA Correspondents bring you cutting-edge coverage from the virtual ASTMH 2021 Annual Meeting
Day 4: Saturday, 20th November 2021
S #85: Malarial Immune Response from Numerous Perspectives
Malaria transmission depends on Plasmodium gametocytes and naturally acquired antibody responses to gametocyte antigens (i.e., Pfs48/45 and Pfs230) which can reduce this transmission. In a study presented by Teun Bousema (Radboud University Medical Centre, the Netherlands) anti-gametocyte immunity was assessed by serological analysis and functional standard membrane feeding assay (SMFA) using plasma samples collected regularly from 433 cohort participants residing in Tororo, Uganda. Malaria transmission declined over the 6-year study period due to highly effective prevention. Bousema presented preliminary results indicating a high prevalence of antibodies against Pfs230 (39.5% in children under 5, 65.3% in 5-11 years, and 84.9% >18 years). For Pfs48/45, antibody prevalence was 13.4%, 44.6%, and 76.0%, respectively. Compared to parasite-free individuals, anti-Pfs48/45 antibody prevalence was higher in those with sub-microscopic infections, microscopically-detected infections, or microscopically-detected gametocytes. Eleven samples out of 263 fully blocked transmission in the SMFA. The surface immunofluorescence assay (SIFA) is particularly discriminative and predictive. Bousema concluded that these results show that anti-gametocyte antibody responses are acquired with increasing cumulative exposure and associated with recent exposure. Intriguingly, age-dependent antibody patterns were not mirrored by an increase in functional transmission reducing immunity. Additionally, SIFA may be a useful tool for SMFA pre-screening and use in epidemiological studies. Ongoing work will better examine the kinetics of functional immunity to gametocytes and may inform the development and deployment of transmission-blocking vaccines.
Jason Nideffer (Stanford University, United States of America) began by showing how males, aged between 5 and 15 years, had a better and longer immune tolerance towards Plasmodium infections in contrast to females of the same age. In the study, 26 female and 23 male participants from Uganda were recruited. His study used flow cytometry, high throughput Luminex-based magnetic bead-based assay, and single-cell transcriptomic RNAseq to assess immunological responses. The data showed that males produced more pro-inflammatory cytokines in response to Toll-like receptor (TLR) stimulation. Interestingly, all participants that were previously exposed to malaria expressed a heterogeneous population of CD123+ myeloid cells, however, this was more in males than in females. The CD123+ cells, commonly associated with plasmacytoid dendritic cell expression, expressed CD11c; a myeloid marker, which significantly altered the myeloid compartment. It was noted that cytokine and HLA-DR cells were expressed consistently by the CD123+ population. The effect of CD123+ myeloid compartments on host immunity revealed inconclusive results, however, females aged 7 – 11 were found to have higher titres of IgG against merozoite surface proteins 1 and 2 suggesting females might have advanced immune responses against malaria and other parasites. Nideffer concluded his talk by expressing his intentions of looking into how CD123+ cells may prime the male adaptive immune response.
Clinton Onyango (University of New Mexico-Kenya, Kenya) spoke about how severe malarial anemia (SMA) has resulted in a high mortality rate in children aged 3-36 months. Low haemoglobin (Hb) has caused inefficient erythropoiesis and numerous innate pathways have been associated with SMA pathogenesis. This study aimed to characterise SMA pathogenesis pathways by sequencing the entire expressed transcriptome of both non-SMA (Hb>7.0 g/dL) and SMA children (HB<6.0 g/dL). An enrichment analysis was conducted to identify the top emerging pathways and the IL-5 signalling via the JAK/STAT pathway was determined as the top-ranked differentially regulated pathway in children’s SMA pathogenesis. IL-5 is involved in several innate and adaptive responses. The results obtained showed that impairments within the IL-5 negative feedback loop can inhibit the JAK/STAT pathway whilst diminished B cell differentiation and maturation and class switch recombination cause an inability to generate appropriate adaptive immune responses. Lastly, Onyango’s results showed that transcriptional analysis suggests that SMA may disrupt processes that inhibit apoptosis.
Isobel Walker (University of Melbourne, Australia) talked about how malaria antibodies are acquired with exposure to infection and age and attack varying parasitic antigens such as Plasmodium falciparum erythrocyte membrane 1 (PfEMP1). These antibodies modulate parasitic binding and sequestration in blood cells and vessels. PfEMP1 has several phenotypes with domains that are associated with severe or uncomplicated malaria (UM). The aim of this study was to determine whether a subset of 33 PfEMP1 domains are associated with cerebral malaria (CM). The study screened antibodies of 98 Malawian children admitted with either CM or UM using a multiplex immunoassay method. The screening aimed to determine the quantity and quality of the fragment crystallizable region (FcR) of antibodies. The FcR may be indicative of the protective functions of the antibody. Walker’s study found that antibodies were able to accurately (83%) predict the clinical outcome of PfEMP1 antigens. She also noted that antibodies that bound to ICAM1 and CD36 binding, as well as rosetting PfEMP1, were associated with UM whilst those that bound to DBLδ1 PfEMP1 types were correlated with CM. IgG2 and IgG4 titres as well as specific antibodies that engaged with FcRs found on monocytes and neutrophils were also associated with UM.
Although Plasmodium vivax is the most common malarial species worldwide, it remains rare in Sub-Saharan Africa. Since P. vivax must bind to the Duffy antigen receptor expressed on host erythrocytes for merozoite invasion, the absence of P. vivax infection may be due to elevated levels of Duffy negative individuals on the continent. Lauren Bradley (Case Western Reserve University, United States of America), presented the results of a study that explores P. vivax naturally acquired immunity in Duffy negative individuals. More specifically, she studied malaria burden, seroreactivity and seroprevalence across Duffy phenotypes, and analysed clustering effects of antibody response on Duffy expression. A cross-sectional survey in low transmission endemic areas in Ethiopia allowed collecting samples that were analysed in serologic assays and sequencing for prevalence of malaria, Duffy phenotype and seroreactivity. Lauren showed that Duffy negativity offers strong but incomplete protection against P. vivax infection. Individual P. vivax antibody development is reduced in Duffy negative people. Duffy negativity correlates with seroprevalence of P. vivax antibodies. Taken together, the data suggest that antibodies against P. vivax are less likely to develop in Duffy negative individuals, reflecting lower infection rates in these populations.
Wael Abdrabou’s (New York University Abu Dhabi, United Arabs Emirates) study aimed at evaluating intra- and inter-ethnic and metabolite expression before and during a Plasmodium falciparum infection. In his study, a mixed methods approach was used to identify metabolites and to conduct assays. Children from two ethnic groups (Mossi and Fulani and Gouin) in Burkina Faso, aged between 5 and 10 years, were recruited with the Mossi and Fulani tribes being more resistant to malaria. After metabolic profiling was conducted, 92 metabolites associated with parasitemia were used to identify the most perturbed metabolic pathways during infection. From these, 12 steroids were identified and revealed to have a positive correlation with parasitemia whilst a negative correlation with lymphocyte levels was noted. These steroids were found to be immunosuppressive as they inhibited T helper cells signalling pathways and activated T exhaustion signalling pathways. To conclude his talk, Abdrabou summarised that P. falciparum induced steroid expression had an immunosuppressive effect on the adaptive immune response T cell functions. However, this would not affect Mossi and Fulani tribes’ children due to the heterogeneity in their steroid expression when infected with Plasmodium. This variability caused a heightened immune response and reduced susceptibility to P. falciparum infection in these tribes.
Fergal Duffy (Seatle Children’s, United States of America) gave a brief overview of how the radiation attenuated sporozoite (RAS) vaccination approach works against controlled human malaria infection. As the immunological correlation of RAS-induced protection was unknown, the study aimed to determine the systemic responses correlated with RAS-induced vaccination. To achieve this, the immunisation with radiation attenuated Plasmodium falciparum sporozoites (IMRAS) trial was conducted on protected and non-protected cohorts. Samples obtained were subjected to whole blood RNAseq and flow cytometry analysis allowing identification of differentially expressed genes and immune cell phenotyping. High inflammation and interferon modules were observed in the non-protected cohort only after the first immunisation. The high inflammation was correlated with a notably high population of monocyte (ILT3+, CD11c) and dendritic cells whilst high baseline levels of another subtype, ILC2, was associated with type 2 immunity was observed. After the first immunisation, immune cell expression fluctuated. On the contrary, the protected cohort displayed a more consistent expression of immune cells throughout the five immunisations. However, Vδ2 γδ-T proliferated in protected and non-protected cohorts and these cells may have a protective function.
S #88: Malaria: Genetics, Genomics and Modeling
Sachel Mok (Columbia University Medical Center, United States of America) discussed her work on the characterization of a novel resistance mediator to the synthetic ozonide, OZ439 (artefenomel), which is an antimalarial pre-clinical drug candidate currently under analysis. It has been found that OZ439 has a long in vivo half-life of 46-62 hours, an advantage over dihydroartemisinin (DHA) which has a half–life of less than 1 hour. She used CRISPR/Cas9 k13 gene editing to validate the K13 A212T mutation which she identified as a new molecular marker of OZ429 in vitro resistance and assessed the impact of the mutation on survival in mutant and wild type parasites. She observed that while K13 A212T did not alter IC50 or IC90 or ring survival rates to OZ439 or DHA, yet parasites with K13 A212T+R539T mutations recovered faster after 48 hours of OZ439 exposure. Proteomics and metabolomics profiling were performed to elucidate whether the A212T mutation may affect haemoglobin (Hb) catabolism and if this is the primary mechanism of lowered antimalarial activity and OZ439 resistance. She found that A212T neither affects Hb processing, nor impacts peptide regulation in response to OZ439 or DHA. Instead, A212T+R539T mutations enhance cell recovery post drug-removal via the upregulation of metabolites linked to redox, lipid, and aspartate metabolism.
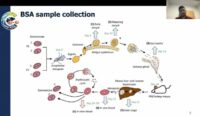
Sudhir Kumar (Seattle Children’s Research Institute, United States of America) began by outlining the importance of carrying out Plasmodium falciparum genetic crosses, including those that had historically identified PfCRT mutations as a driver of chloroquine resistance. Kumar’s group carried out bulk segregant analysis (BSA) to evaluate the change in parental mitochondrial (MT) and apicoplast (Api) genomes by using recombinant progeny pools. They observed a decrease of NF54 MT allele frequency in the cross of NF54xNHP4026 during the transition from sporozoite to liver stages, a phenomenon that had not been seen before. The study concluded that progeny from allopatric crosses differ in MT/Api inheritance while sympatric crosses do not. Further investigation to generate progeny with NF54 MT/Api genomes by crosses of male sterile NF54 and female sterile NHP4026 parasites, as well as to determine if MT inheritance in the recombinant progeny is associated with regions of nuclear genome inheritance will be conducted.
Angela Early (Broad Institute, United States of America) talked about the epidemiological history of P. falciparum (Pf) in the Pacific Coast Region of South America. She presented the genomic profiling of Pf on Colombia and Ecuador Pacific Coast. They analysed 161 whole genome sequences from monoclonal infections sampled in Colombia and Ecuador collected between 2013 and 2016, where 60% and 10% of malaria infections are Pf, respectively. Sequencing confirmed high relatedness and low haplotypic diversity. Six of these clusters have persisted for at least 10 years, but the population as a whole is not static, as there is evidence of de novo mutations. She analysed chromosomal segments between pairs of samples and identified five putative crosses among 11 of the 17 genomes sampled after 2012. Two of these crosses generated novel combinations of drug-resistance haplotypes. She coupled this with epidemiological data and found that the timing of crosses likely coincided with a period of high transmission. She observed that the recombination patterns support a hypothesis of outbreak-driven outcrossing and generated new combinations of resistance markers. Using identity-by-descent (IBD) analysis, she confirmed the decades-long persistence of Pf clones along the pacific coast of Colombia and Ecuador.
Jessica Ribado (Institute for Disease Modeling, United States of America) described epidemiological and genetic models of malaria transmission to understand transmission history and features of transmission. At the onset of her presentation, she stated that models help us take what we know about an environment and turn it into actionable information. In her analysis, she combines EMOD, a detailed stochastic agent-based model for simulating malaria genetic epidemiology with Genepi to give information about each unique strain in an infection, and feeds into an observational model. Observational models can interrogate variant properties of sampled infections that recover modeled transmission metrics. By comparing estimated metrics in modeled truth, she can derive genetic metrics that are most informative about malaria transmission to guide operational decision-making. Her findings suggest that simulated data provides a tool to understand how observations impact relationships between genetic and epidemiological metrics. Modelling can support the use of genomic data in productive ways to achieve elimination.
Joshua Suresh (Institute for Disease Modeling, Bill & Melinda Gates Foundation – BMGF, United States of America) investigated the impact of using primary schools to deliver intermittent preventive treatment to school-aged children (IPTsc) using mathematical models of transmission. Models were applied in Zambia, which represents a southern archetype, and Burkina Faso, representing a Sahel archetype. Their modeling found that IPTsc shows strong direct and community impact. An IPTsc strategy of presumptive treatment of all students with dihydroartemisinin-piperaquine (DP) once per school term reduces burden in school-age children by about 50%, and reduces the overall clinical burden in the community by about 30%, with the greatest community benefit found at the lowest transmission intensity. IPTsc outperforms increasing ITN coverage from 70% to 90%, which only reduces overall clinical burden by about 10%. This measure combined with standard seasonal chemoprevention (SMC) is less expensive and reduces more cases than SMC alone. He also presented important model factors which are not being evaluated including potential benefits like improved educational outcomes for school age children or potential risks like acceptability of frequent drug dosing to older children.
Branwen Owen (Swiss Tropical and Public Health Institute – Swiss TPH, Switzerland) aptly began with a call to be bold, highlighting the need to adapt interventions in order to reach new heights in the fight against malaria. Owen and team modeled data from an implementation trial that was conducted in Karamoja, Uganda. This trial used or planned to use five rounds of SMC in children less than five years old, from 2021 and 2022. Karamoja was considered suitable because of its high malaria prevalence, low bed-net coverage, single annual rainy season, and a mainly nomadic population. The model assumed 80% coverage at each of the five rounds, and predicted marked reduction in malaria incidence compared to a scenario of no SMC. Owen pointed out that the model shows a greater effect in incidence reduction when baseline incidence is low, and deviations to the plan are less important when malaria incidence is high. Therefore, it is vital to understand baseline incidence or prevalence at the site of, and to identify and eliminate likely impediments to maintaining coverage throughout SMC implementation. Owen, however, reminded the audience that a model can only be as accurate as its input parameters and assumptions.
S #93: Intermittent Preventive Treatment for Malaria in Infants with Sulfadoxine – Pyrimethamine (SP-IPTi): Fit for Purpose in 2021?
David Schellenberg (Global Malaria Program, WHO) chaired the session and gave a detailed background of how the SP-IPTi strategy was developed and the important factors influencing IPTi effectiveness such as access, coverage, incidence and drug efficacy. Professor Schellenberg highlighted that WHO recommended strategies should be tailored to fit each country’s circumstances and that SP-IPTi was no different. Contact points for SP-IPTi can extend into the second year of life, and be delivered at any convenient health system touch point depending on the country specific setting.
Clara Menéndez (Barcelona Institute for Global Health – ISGlobal, Spain) began her presentation by giving a historical update on intermittent preventive treatment for malaria in infants (IPTi). The first trial with IPTi using sulfadoxine-pyrimethamine (SP-IPTi) was conducted in 2001 in Ifakara, Tanzania. The positive results resulted in the creation of the IPTi Consortium with the support of Bill and Melinda Gates Foundation (BMGF) which analyzed the results of 6 randomised, placebo control trials in 8 different countries. The metanalaysis showed that SP-IPTi was effective against clinical malaria (30.3%) and anemia (21.3%) in addition to reducing hospital admissions with parasitemia (38.%) and all-cause admission (22.9%). In 2010 the World Health Organisation (WHO) recommended IPTi and recently these recommendations have been revised. However, to date, Sierra Leone is the only country that has implemented IPTi despite the challenges and barriers. Menedez later presented an overview of a new 40 months MULTIPLY project being implemented in Sierra Leone, Togo and Mozambique with the support of the Ministries of Health of African malaria endemic countries. This pilot implementation project MULTIPLY will expand SP-IPTi into the second year of life and will increase the uptake delivered along with the expanded immunization programme (EPI) mobile-outreach clinics. She concluded that it’s high time to reconsider implementation of SP-IPTi in endemic countries and to overcome the already known health system barriers.
Dorothy Kah Fosah Achu (National Malaria Control Program – NMCP, Cameroon) presented the policy consideration for interventions for malaria in Cameroon. Malaria is a major public health problem in the country with increased incidence since 2016. The parasite accounts for 29.1% of outpatient visits, 40% hospitalizations, and 64% of deaths mostly among children. These findings inform that there is a need to target this vulnerable group with effective interventions. Control strategies adopted in the national strategic plan (NSP) 2019-2023 are preventive; through vector control, using long-lasting insecticidal nets (LLINs) given to children under-5 through EPI, chemoprevention using Intermittent preventive treatment for infant (IPTi-SP) with sulphadoxine-pyrimethamine; and case management through systematic diagnosis using RDT/microscopy and treatment of both uncomplicated and severe malaria cases in health facilities and via community case management. She then explained their rationale for including SP-IPTi in their NSP to reduce malaria morbidity and mortality in infants and children. With the goal of preventing malaria in vulnerable groups, they will implement SP-IPTp. However, attention should be given to cost-effectiveness, feasibility, equity of services, supplies and acceptability of SP-IPTi intervention.
Olusola Oresanya (Malaria Consortium, Nigeria) presented the context for the rationale of implementation research for SP-IPTi in Nigeria. Although Nigeria has witnessed a decline in malaria prevalence from 42% to 23% between 2010 and 2018, malaria remains a public health challenge by contributing 25% to the malaria global burden. Between 2013 and 2018, infant mortality rate only declined from 69% to 67%, thus flagging the need for more tailored intervention for this age group. SP-IPTi is currently not implemented in Nigeria, but an operational study (2020-2024) is part of the National Malaria Strategic Plan (NMSP) and will assess clinical effectiveness using cRCT (arm one SP-IPTi 3 doses; arm two SP-IPTi 5 doses; and arm three control with standard of care). It will also provide an opportunity to assess its operational feasibility using mixed-method study. She concluded her presentation by stating that evidence generated will inform policy formulation and adoption of the intervention by removing the bottlenecks.
Sian Clarke (London School of Hygiene and Tropical Medicine – LSHTM, United Kingdom) presented a co-creation design approach with the national stakeholders in the countries exploring the opportunities for adaptation of IPTi+ to fit country needs and SP resistance in sub-saharan africa. The project will be implemented and evaluated in 4 countries Ivory Coast, Benin, Cameroon and Mozambique; and later with more research and policy adoption in Ghana, Zambia and the Democratic Republic of the Congo. The + in IPTi, means to expand the number of doses given in IPTi, from three up to eight doses, and to widen the age range to be able to give the chemopreventive treatment during the second year of life. Countries will design their own strategy through a process called co-design. Model implementation for each specific country will be evaluated by research studies to examine the process of implementation, impact achieved, cost and cost effectiveness. This will Inform countries on implementation, adaptation of IPTi in policy, and for World Health Organisation development of guidelines. Clarke mentioned that they will also assess the effect of SP resistance by conducting cohort studies in Mozambique; evaluate the effect of different genotype mutations (including DHPS 581G mutation) in Cameroon and Zambia, and lastly genotyping surveys in countries of interest to determine subnational prevalence of genotype maps. Clarke concluded her talk by stating that findings from this project will help in development of a decision support tool for countries wanting to implement SP-IPTi+ and choosing the appropriate delivery strategy.
S #97: Malaria: Innovations in Malaria Prevention and Control
Baltazzar Candrinho (National Malaria Control Program – NMCP, Mozambique) started with an overview of the national malaria strategic plan in Mozambique between 2017 and 2022, and its recommendation of seasonal malaria chemoprevention (SMC) as a strategy to reduce malaria burden in high risk locations. In partnership with Malaria Consortium, the NMCP conducted an implementation study aiming at determining the protective effect of sulfadoxine-pyremethamine + amodiaquine (SPAQ) when used for SMC, and to assessthe feasibility and accessibility of implementing SMC. The non-randomized controlled trial adopted a standard SMC implementation model commonly used in West and Central Africa. Candrinho reported that a high coverage of SMC knowledge, delivery and evidence of receipt of SPAQ was observed. Confirmed malaria cases during follow-up reduced significantly in intervention districts compared to control districts. Study also showed that SMC implementation using SPAQ is safe, feasible, and highly acceptable in the study areas. There was no report of serious adverse events. Parasite resistance to SP was high while resistance to AQ was low. He concluded by stating that the team’s research plan for the second phase of the study.
Jimmy Anzolo (PATH, the Democratic Republic of the Congo) presented the Emergency Operations Centers (EOCs)-Malaria project.EOCs are used to follow emerging crises i.e. COVID-19. Anzolo described how PATH engaged with the Ministry of Health in the Democratic Republic of the Congo (DRC) to support its EOC’s engagement to respond to COVID-19. The aim was to strengthen malaria burden reduction efforts while increasing the EOC’s efficiency in responding to other emergencies. To reinforce the EOC’s capacity to monitor multiple diseases, a dashboard was created. It now allows tracking morbidity and mortality data for COVID-19 and other diseases with epidemic potential. In the same approach, malaria dashboards were developed and are used by the National Malaria Control Program to monitor key indicators and cover existing malaria data sources and gaps. DRC malaria data served to calibrate a model to stratify malaria interventions. Predictive modeling helps outbreak detection, allowing a timely response. Anzolo also presented their training activities on malaria surveillance and management and the use of digital COVID-19 tracking tools. The next steps in this project aim to reinforce the use of data by the health system and to enable visualization down to the health area level; as well to continue enriching the dashboards using additional data sources.
Hillary Topazian (University of North Carolina at Chapel Hill, United States of America) discussed the effectiveness of a national mass distribution campaign of long-lasting insecticide-treated Nets (LLINs) and indoor residual spraying (IRS) on clinical malaria in Malawi during the period 2018-2020. Topazian started emphasizing that bednets are the backbone of Malawi malaria interventions but neither its lifespan nor efficacy has been properly determined in field settings. Bed nets are distributed through massive campaigns every 3 years, and several insecticides are used. Topazian’s group tried to determine how a mass distribution campaign of LLINs changes malaria risk over time. District Health Information Software 2 (DHIS2) data was used to determine the effect of LLIN type or annual application of IRS. After massive distribution of LLINs and IRS before high transmission seasons, malaria risk decreased from 25.6 to 16.7 cases per 100 people from 2018 to 2019, but rebounded to 23.2 in 2020, resulting in significant risk differences of -8.9 in 2019 and -2.4 in 2020 as compared to 2018. This shows that LLINs have a reduced efficacy lifespan once in the field; also, Piperonyl butoxide-treated (PBO) LLINs were more effective than pyrethroid-treated LLINs. DHIS2 probes to be a valuable source of information to follow malaria trends and to evaluate malaria trends and ongoing interventions.
Ann-Sophie Stratil (Malaria Consortium, Mozambique) commenced by stating that the outcome of two systematic assessments of Mozambique’s surveillance system conducted in 2016-2018 identified the need for integrated Malaria Information Storage System (iMISS). Hence, the National Malaria Control Programme (NMCP) in partnership with other stakeholders initiated the development of iMISS. The primary goal of iMISS was to enable malaria staff at all levels of the health system to monitor key indicators, and to provide quality evidence to plan and implement responses. Stratil’s study focused on evaluating the outcomes of the iMISS roll-out at the health facility level. Expected outcomes included data quality assessment, adoptability, acceptability, and maintenance issues during the first six months after roll-out. Quantitative and qualitative approaches were used to collect data using questionnaires and interviews. Findings indicated that iMISS is effective and well accepted. Data quality is sufficient, and the number of maintenance issues reported decreased over time. However, gaps were also identified. Technical issues need to be resolved while resolving the time of maintenance issues and adoption/data use needs improvement. She concluded by enumerating the next steps on integrating lessons learnt to allow the iMMIS reach its full potential before nationwide roll-out at the health facility level.
Adefisoye Adewole (African Field Epidemiology Network, Nigeria) presented the lessons learnt from the implementation of the Malaria Frontline Project (MFP) in the states of Zamfara and Kano in Nigeria between2016 and 2019. Adewole stated that poor data quality is a problem in all monitoring systems and limits decision making. A collaboration between the United States of America’s Centers for Disease Control and Prevention (CDC) and the Nigeria National Malaria Elimination Program (NMEP) allowed the establishment of the Malaria Frontline Project. For capacity building, the strategy developed by the National Stop Transmission of Polio was applied. During the project, a local government area (LGA) supervisory team analyzed testing rate, clinical diagnosis and directly observed intermittent preventive treatment (IPTp) from the District Health Information Software 2 (DHIS2). Health facilities (HF) performing low in those indicators were visited more frequently, conducting on-the-job training and mentoring during visits. Adewole’s group analyzed the reports of support visits to HFs from 2017 to 2019. During MFP, HFs testing rates increased and clinical diagnosis decreased. The proportion of HFs practicing the recommended directly observed IPTp increased. HFs analyzed the selected indicators from their malaria monitoring chart and used the results to make program decisions. DHIS2 data analysis helped HCWs at HF and LGA levels to improve malaria program performance.
Alassane Dicko (Malaria Research and Training Center, Mali) presented background information on seasonality of malaria and effectiveness of seasonal malaria chemoprevention (SMC). Despite the high efficacy of SMC, malaria continues to be a burden in Mali which is an indication of the need for additional intervention tools such as the malaria vaccine. The rationale for using the RTS,S malaria vaccine for seasonal vaccination was because of its high efficacy for a few months before it declined, and the possibility of restoring its efficacy with a booster vaccine. Alassane stated that their study was conducted among children 5-17 months old resident in Mali and Burkina Faso using a double-blind randomized control trial design. Morbidity data were collected continuously over 3 years through passive surveillance. Findings showed a very high vaccine and SMC coverage among the study groups throughout the period of study. The protection provided by seasonal RTS,S vaccination against clinical malaria was not inferior to the protection provided by 4 cycles of SMC per year. The addition of RTS,S on top of SMC resulted in superior protection compared to the protection provided by SMC alone. And no major safety issue was recorded.
S #102: Ex Vivo Assessment of Drug Susceptibility of the Antimalarial Drug Pipeline
Oriana Kreutzfeld (University of California, United States of America) presented work on the efficacy of antimalarials (exploratory and advanced compounds) on fresh clinical isolates from Tororo and Busia Districts in eastern Uganda from 2016 to 2020. The long and ongoing study was done to evaluate the full Medicines for Malaria Venture (MMV) pipeline and identify genomic markers associated with decreased susceptibility to newly developed inhibitors. Fresh isolates from the patients diagnosed with Plasmodium falciparum (Pf) infection were collected and blotted on filter paper for genotypic analysis. Ex vivo drug susceptibility studies were done on samples with parasitaemia greater than 1%. She reported parasites being highly susceptible to most of the 40 MMV pipeline inhibitors; similar results were seen in a newer study done in Bobo-Dioulasso, Burkina Faso. PfATP inhibitors were highly active against Uganda Pf isolates. Isolates with the G223S mutation had decreased susceptibility to PfATP inhibitors, but differences were modest. Older PfDHR inhibitors (pyrimethamine and cycloguanil) had reduced activity against Ugandan Pf isolates with multiple PfDHFR mutations, whereas a 2, 4 – diaminopyrimidine (P218) was highly active. Surprisingly, 16% of isolates carried the quadruple 51I/59R/108N/164L DHFR mutation, which had decreased susceptibility to pyrimethamine, cycloguanil and P218, although P218 retained excellent activity.
Laurent Dembelé (Malaria Research and Training Center of Bamako, Mali) presented work on the characterization of ex vivo malaria parasite drug susceptibility in Ghana and Mali. He developed an ex vivo assay to test drug activity against Plasmodium falciparum (Pf), malariae (Pm), ovale (Po), and vivax (Pv) species, while a polymerase chain reaction assay was used to confirm the species identified. Using fresh isolates from a pilot study, the activity of a reference drug was established. In Mali, Pf was the most predominant species, while Pm accounted for 15% of the infections. He reported a similar trend in Ghana, where Pm was the second most prominent amongst the 22% non-Pf infections. A decreased susceptibility of Pm isolates to artemether, lumefantrine and chloroquine was observed compared to Pf, but no differences in susceptibility to artesunate, lumefantrine, chloroquine, quinine and pyrimethamine were observed in Po. Piperaquine was found to be a potent inhibitor of Pm and Pf isolates. Furthermore, all species were susceptible to novel candidate antimalarial drugs – KDU691 and GNF179 – with no significant differences among the species in Ghana and Mali. He suggested further research to be done to assess the efficacy of artemisinin combination therapies and other drugs against Pm infections.
Camille Roesch (Institut Pasteur in Cambodia) presented on profiling new antimalarials against Cambodian parasites. Pf strains in Cambodia are associated with clinically relevant resistance to ACTs. Parasites in South-East Asia are polymorphic and versatile so it’s crucial to screen new molecules against representative strains that are circulating. Currently, there are two major profiles of resistance detected in Cambodia: MQ-R and PQ-R. For sustainable malaria control, they proposed a triple combination therapy, although triple resistance may exist. Within their strains and molecules tested, four out of nine drug candidates would be accepted because they had similar IC50’s among all genotypes and their activity was not associated with pre-existing resistance patterns. Another four molecules would be rejected because their IC50’s were associated with preexisting resistance patterns. Ongoing work includes whole genome sequencing of isolates with phenotypes of interest.
Caroline Aguiar (University of Sao Paulo, Brazil) talked about ex vivo assessment of drug susceptibility of the antimalarial drug pipeline against Pf and Pv using a novel schizont maturation assay. To analyze, they counted the number of schizonts and compared results with those from non-treated controls. They tested over 100 compounds using the schizont maturation assay against Pv and Pf. Importantly, no significant differences in susceptibility between Pf and Pf were observed for the antimalarial used in the field (lumefantrine, chloroquine, mefloquine, artesunate, pyronaridine, atovaquone, piperaquine and amodiaquine). A good correlation was found between IC50 for Pf and Pv field isolates, showing that most compounds that are active against Pf are also active against Pv parasites.
S #113: Genetic Approaches to Elucidating Plasmodium Falciparum Antimalarial Modes of Action, Drug Resistance and Fitness
Sachel Mok (Columbia University Irving Medical Center, United States of America) discussed a study titled, “Mapping antimalarial drug resistance determinants using P. falciparum genetic crosses in humanized mice”. The presentation focused on phenotypic, genetic and genomic analyses that have mapped determinants of parasite resistance to multiple antimalarials such as artemisinin, piperaquine, chloroquine and quinine, using contemporary drug-resistant clinical isolates obtained from Southeast Asia crossed with the African NF54 strain. Her findings provide compelling evidence that the k13 gene is the primary mediator of ring-stage parasite resistance to artemisinin derivatives and suggest the presence of secondary determinants that might modulate survival rates among the k13 mutants. Also, piperaquine was observed to select for the Southeast Asian KeI1/Pla1 co-lineage. Forward and reverse genetic studies highlighted an epistatic interaction between the Pf chloroquine resistance transporter (PfCRT) Dd2+M343L variant and plasmepsin II gene amplification in contributing to piperaquine resistance. This group also identified the drug metabolite transporter (DMT1) as a new potential marker for quinine resistance. Overall, the studies showed that quantitative trait locus (QTL) mapping can identify loci involving drug resistance, which may influence the parasite’s fitness during resistance acquisition. Finally, her data showed that genetic crosses are a useful tool for teasing out drug resistance mechanisms, including for drugs with unknown modes of resistance or action, through the use of bulk segregant analysis (BSA) and clone-based linkage mapping.
Ashley Vaughan (Seattle Children’s Research Institute, United States of America) discussed the leveraging of Plasmodium falciparum genetic crosses to understand fitness and life cycle progression. P. falciparum genetic crosses are now routinely carried out in the lab and along with collaborators, six unique crosses with multiple biological replicates spanning eight parental parasites have been conducted over the last four years. The team is using bulk segregant analysis (BSA) to understand nutritional genomics by comparing the growth of recombinant parasite populations in different media formulations in long-term competitive fitness experiments. In vitro P. falciparum cultures often use AlbuMax as a lipid substitute for human serum, however, the impact of using the latter on parasite growth and drug sensitivity is poorly understood. Analysis of genome inheritance over time in serum versus AlbuMax revealed strong genomic skews on chromosome 13, in a segment containing erythrocyte binding antigen 140 (EBA-140), suggesting that redundant invasion pathways used by parasites are dependent on the nutritional status of the media. Further work will determine the relevance of these studies to the in vivo condition. The team has also used BSA in combination with classical genetics to show that mutations in the amino acid transporter pfaat1 are linked to resistance to the antimalarial chloroquine and mutations in the chloroquine resistance transporter pfcrt.
Marcus Lee (Wellcome Sanger Institute, United Kingdom) presented a study that focused on the use of barcoding technology to define parasite resistance to experimental antimalarials that are being explored for future drug discovery research. There is a need to develop new drugs that are not subject to existing resistance mechanisms. To achieve this, a relatively high proportion of Plasmodium genes are required for normal growth, which suggests many potential drug targets remain to be associated with chemical inhibitors. The study utilized resistant parasite lines generated by the Bill & Melinda Gates Foundation-funded Malaria Drug Accelerator (MalDA) consortium that aims to identify new drug targets, understand modes of action and generate early lead inhibitors. Resistant lines were barcoded by CRISPR, allowing multiple lines to be pooled and grown in competition. Barcode sequencing of the parasite pool can reveal fitness and drug response phenotypes. Fitness assays measured growth over 60 days and revealed a range of fitness costs for different mutants. In addition, short term drug exposure of the pool allowed rapid target identification by detection of enriched barcodes. Barcode enrichment is reproducible and the assay can also be configured to detect hypersensitivity of specific mutants to test compounds.
Charisse Flerida Pasaje (Massachusetts Institute of Technology, United States of America) showcased how she utilized conditional gene down-regulation studies and developed a cell-based differential sensitivity phenotypic platform to inform antimalarial drug discovery by validating compound-target interactions and screening for inhibitors of an essential protein. To expand on her work, she reported on a number of applications that were carried out as part of the MalDA consortium by demonstrating the ability of the platform to validate inhibitors of the cytosolic isoleucyl-tRNA synthetase, the cGMP-dependent protein kinase PKG, and the bifunctional farnesyltransferase/ geranylgeraniol pyrophosphate synthase (GGPPS). Additionally, she showed the specificity of the technology to pick up compound-target pairs when screening against panel conditional knockdown lines. Key concluding points in her presentation were that conditional knockdown systems allow for efficient and accurate mapping of target-compound interactions, and can confirm or reject resistance mechanisms that emerge from various target identification pipelines.
This report is brought to you by the MESA Correspondents. Senior editorial support has been facilitated by the Organizers and Co-Chairs of the symposia and Divya Beri (Lindsley F. Kimball Research Institute, New York Blood Center, USA). This report is cross-posted on the MESA website and on MalariaWorld.
Published: 24/11/2021
This report is brought to you by the MESA Correspondents Edima Ottoho, Tope Kayode, Franklin Tembongshu Formilack, Lucy W. Mwangi, Vita Mithi, Ana Alonso, Faith Hungwe, Olajoju Temidayo Soniran, Isabelle Delrieu, Patricia Doumbe Belisse, and Carlos A. Fernández Miñope. Senior editorial support has been facilitated by the Organizers and Co-Chairs of the symposia and Divya Beri.
THEMES: Basic Science | Epidemiology | Health Systems