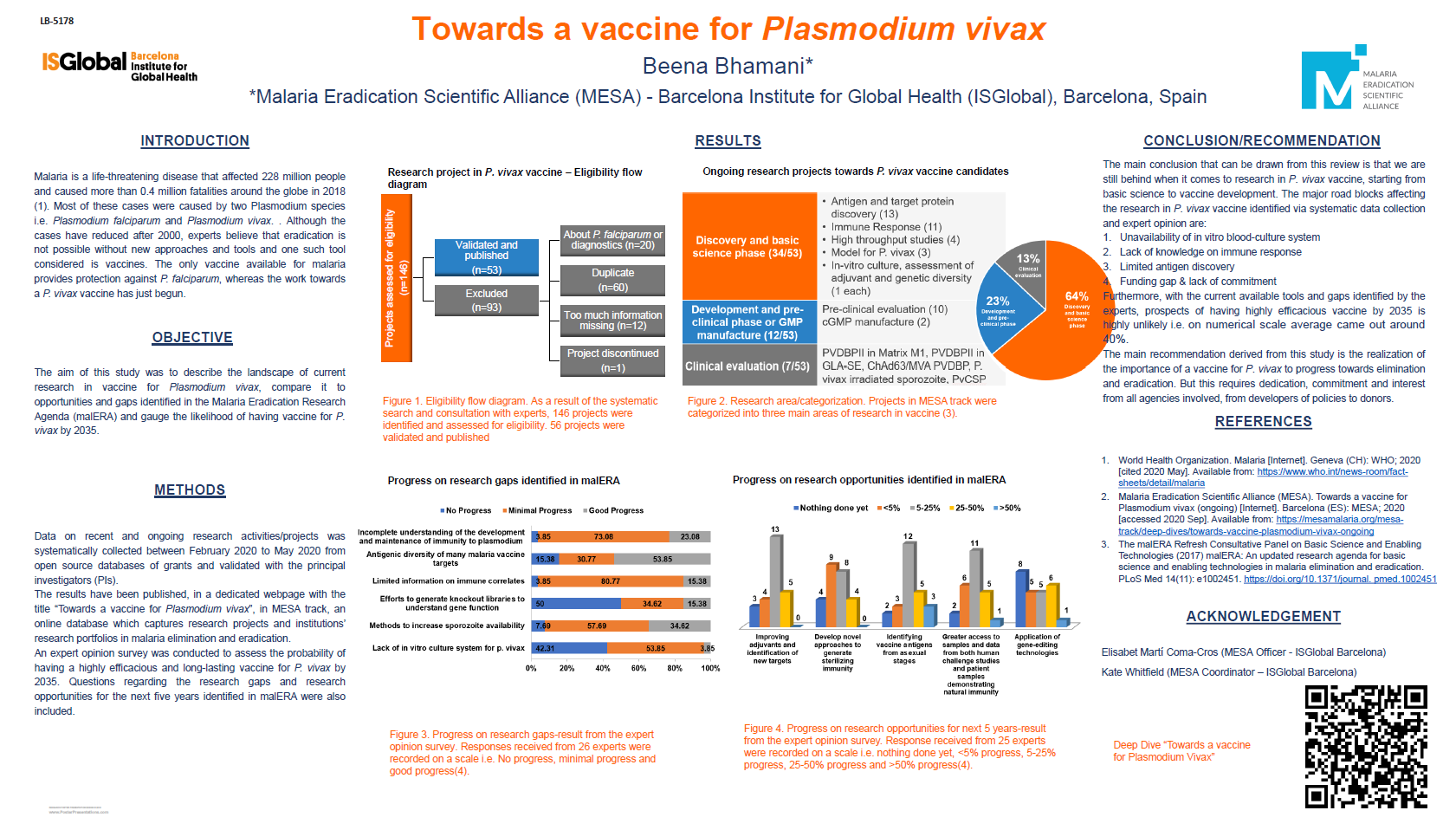
70th ASTMH Virtual Annual Meeting – 2021: Day 2
Thursday, 18th November 2021
Published: 18/11/2021
This report is brought to you by the MESA Correspondents Edima Ottoho, Tope Kayode, Franklin Tembongshu Formilack, Lucy W. Mwangi, Vita Mithi, Ana Alonso, Faith Hungwe, Olajoju Temidayo Soniran, Isabelle Delrieu, Patricia Doumbe Belisse, and Carlos A. Fernández Miñope. Senior editorial support has been facilitated by the Organizers and Co-Chairs of the symposia and Divya Beri.
THEMES: THEMES: Basic Science | Epidemiology | Health Systems
MESA Correspondents bring you cutting-edge coverage from the virtual ASTMH 2021 Annual Meeting
Day 2: Thursday, 18th November 2021
Symposium #12: Entomological Data to Guide Strategic Deployment of New Types of Insecticide Treated Nets for Control of Pyrethroid Resistant Malaria Vectors
Amy Lynd (Liverpool School of Tropical Medicine, United Kingdom) started her presentation by giving background information on the importance of piperonyl butoxide-long lasting insecticide nets (PBO-LLIN) which combine pyrethroids with PBO. She provided an overview of the study which was a cluster randomised trial (CRT) conducted in Uganda following the nationwide distribution of LLINs with and without PBO. The primary outcome was to evaluate parasite prevalence by microscopy in children aged 2-10 years and secondary outcomes measured prevalence of molecular markers associated with insecticide resistance and LLIN coverage, survivorship, durability, bioefficacy and chemical content. Baseline survey showed that LLIN ownership and coverage had reduced markedly, parasitaemia and anaemia varied widely by region, and Anopheles gambiae s.s. was the predominant vector across all areas. At the end of the clinical trial, Lynd’s group found that PBO-LLINs were significantly better at reducing parasitaemia and vector density than LLINs without PBO up to 25 months after distribution; decreased net coverage was associated with an increase in parasitaemia after 25 months; and housing type was an important risk factor for parasitaemia, vector density and net damage.
Joseph Chabi (U.S President’s Malaria Initiative – PMI, VectorLink, United States) commenced his presentation by stating that malaria-endemic countries had distributed insecticide treated nets (ITNs) over the past two decades with little consideration to insecticide resistance monitoring data because the only available ITNs were pyrethroid-only nets. He also mentioned the pyrethroid resistance status across Africa which had led to the development of ITNs with combination of insecticides such as PBO synergist and chlorfenapyr. Hence, the U.S President’s Malaria Initiative (PMI) VectorLink monitors resistance in focus countries with the vision to allocate resources appropriately to maximize impact of vector control tools. In these African countries, data on vector surveillance and resistance is collected from selected sentinel sites. Available data showed that PBO synergist and chlorfenapyr are effective at killing malaria vectors across PMI countries. In conclusion, he mentioned that several countries have introduced the use of new types of ITNs and PMI still follows up on durability monitoring of the ITNs distributed across selected countries to continuously support and advise National Malaria Control Programs (NMCPs).
Ellie Sherard-Smith (Imperial College London, United Kingdom) presented a systematic review on an evidence-based framework to support selection of the most appropriate malaria vector control intervention in “location”. The role of outdoor biting behaviours and residual transmission, IRS products, and mosquito net products in interrupting the transmission of malaria parasite was assessed using data from cluster-randomised control trials. Majority of the studied population stayed indoors and in bed at night. Anopheles gambiae, An, arabiensis and An. funestus were mostly active at night both indoors and outdoors. Residual transmission was geographically different with mosquitoes species having preferences to bite people, but this was not always related to human blood meals. However, mosquitoes were found to be resistant to insecticides and the entomological impact of mosquito nets showed that mosquitoes became more resistant to insecticides over time in Africa though the timing of the various trials differed and with varying seasons. The future perspective from the study is to use validated interventions in location-specific settings thereby reducing cost and increasing effectiveness of specific vector control mechanisms.
Constant Gbalegba (National Malaria Control Programme, Ivory Coast) presented entomological baseline data collected in 2019 on the new generation of ITNs that has been widely distributed in Ivory Coast between 2016-2020 with support of PMI VectorLink project. He discussed the various monitoring sites with indoor residual spray (IRS) and/or without IRS, insecticide resistance monitoring (IRM) sites and the entomological methods used in the study. With respect to insecticide resistance, he observed high pyrethroid resistance in all the monitored sites and PBO not restoring pyrethroid susceptibility. On the other hand, susceptibility to Chlorfenapyr, pirimiphos-methyl and Clothianidin varied across the sites. Vector human biting rates (HBRs) were high in Sakassou but low in Nassian, while infectivity and entomological inoculation rates also varied in the two study areas. These findings had implications for decision making for distribution of new generation ITNs and standard nets, and selection of IRS sites.
Symposium #15: Late-Breakers in Malaria
Both heme (a host-derived factor) and Histidine Rich Protein 2 (HRP2, a parasite-derived factor) released from lysed parasite erythrocytes contribute to human cerebral malaria (HCM) complications. Adriana Harbuzariu (Morehouse School of Medicine, United States of America) presented the results of an investigation into the pathways mediating heme and HRP-2-induced brain damage. She and her team showed that HRP2 reduced induced pluripotent stem cell (IPSC) proliferation and increased cellular apoptosis, necrosis and inflammation. Heme and HRP2 increased Toll-like receptors 1 and 2 (TLR 1 & 2) gene and protein expression in brain organoids, and monoclonal antibodies against TLR1 and TLR2 reduce the effects of heme and HRP2, thereby demonstrating a TLR1/2 dependent mechanism of action. The up-regulation of TLR2 expression by heme and HRP2 is also observed in the cortex of ECM (experimental cerebral malaria) mice and in the post-mortem brain cortex sections from HCM patients, suggesting that it may play an important role in scavenging of heme and HRP2 in the perivascular space as well as in malaria-induced brain inflammation. However, circulating Neuregulin-1 (NRG1; cytoprotective) declined in HCM, whereas tissue expression of NRG1 and its receptor ErbB4 in damaged brain areas were elevated. The findings show that heme and HRP2 effects are attenuated by exogenously augmenting the Neuregulin-1 a protective factor (NRG1). A very hopeful observation is that adjunctively administering NRG1 with Artemether in ECM mice eliminated parasite burden, reduced brain damage and ECM mortality.
The whole genome sequencing (WGS) of clinical isolates of Plasmodium falciparum is challenging due to low complexity sequences, multi-gene families, extensive nucleotide diversity, tandem repeats and indels majority variation, as well as polyclonal infections and low parasitemia. Karamoko Niare (Brown University, United States of America) presented the development of a new variant calling pipeline for Plasmodium falciparum sequencing based on the Genome Analysis Toolkit (GATK). Firstly, parameters that control the heterozygosity, ploidy, assembly region and read quality were tuned. In addition, a high-quality training dataset was generated to filter false variants out. The GATK4-generated training Variant Call Format (VCF) shows high quality variants. When comparing the outputs to the gold-standard call-sets, the sensitivity of the new pipeline on simulated mixed infections is higher, reaching 71-84% for single nucleotide polymorphisms (SNPs) detection and 66-85% for indels in exons detection within the core genome. The sensitivity is higher than current pipelines for monoclonal infections, reaching 85-92% for SNPs and 88-90% for indels in the case of high-quality Illumina read data, and 78-80% for SNPs and 76-83% for indels in the case of low-quality reads. The precision for SNPS and indels is also higher than that observed for current pipelines: above 96% regardless of the read quality for SNPS, and between 93 and 95% on high quality reads and 76 to 93% on low-quality reads for indels. This new variant calling pipeline provides more accurate variant data. However, when applied to a large WGS dataset it can facilitate population genomics analysis that will serve as evaluation and identification of targeted interventions against the disease.
Resistance to first-line antimalarial piperaquine (PPQ) is observed in Southeast Asia and has been shown to be mediated by mutations in the drug efflux transporter PfCRT. Laura Hagenah (Columbia University in New York, United States of America) works with the team of David A. Fidock and they investigated whether the increasing usage of dihydroartemisinin-PPQ therapy in Africa could lead to the spread or the emergence of PPQ resistance in the continent. The most common chloroquine (CQ)-resistant pfcrt alleles in Africa were edited into the Dd2 Plasmodium parasite strain, and three PPQ-resistant PfCRT mutations seen in Southeast Asia were subsequently edited into these modified parasites. PPQ survival assays show that the F145I mutation in PfCRT confers high-grade PPQ resistance. The T93S mutation confers increased rates of survival at low concentrations of PPQ (~25 nM) in modified parasites. Resistance assays on other antimalarials and common combination therapy partner drugs were also presented. Results showed that PfCRT point mutations T93S, F145I, and I218F result in reduced degrees of resistance to CQ and its metabolite monodesethyl-chloroquine (md-CQ) on both GB4 and CAM783 haplotypes. The F145I mutation on GB4 PfCRT confers increased susceptibility to monodesethyl-amodiaquine (md-ADQ). These results suggest that increased dihydroartemisinin (DHA)-PPQ usage for seasonal malaria chemoprevention in Africa may result in PfCRT mutations that mediate resistance to PPQ.
Jack Hutter (Walter Reed Army Institute of Research in Silver Spring, United States of America) presented results from an expanded assessment of safety and immunogenicty of FMP014, a malaria vaccine candidate.FMP014 is composed of 60 monomers oligomerised into self-assembling protein nanoparticles (SAPN). Each monomer consists of CSP – representing the 3D7 strain of Plasmodium falciparum – that includes the C-terminus, 6 NANP repeats, and 2 CD4 viral epitopes. FMP014 is adjuvanted with the Army Liposomal Formulation containing QS-21 (ALFQ). Safety and immunogenicity were evaluated in ten U.S naïve adult subjects divided into 2 groups: group 1 receiving 20 μg FMP014 and 0.5 ml ALFQ, and group 2 receiving 40 μg FMP014 and 1.0 ml ALFQ, according to a 3-dose regimen (vaccinations: day 0, 28, and 56). Local solicited adverse events were mild and resolved within 72 hours. Systemic solicited adverse events were mild or absent in the low dose group and moderate in the high dose group. No related serious adverse events (SAEs) were observed. Assessment of serum antibodies by ELISAs to full length PfCSP, peptides containing (NANP) 6 and a C-terminal peptide, Pf16, both antibody titers and avidity indices increased after each dose. C-termiuns specific antibodies were dominant by a log over the anti-NANP response. A range of assays provided information on cellular responses induced by FMP014/ALFQ and specifically cytokine profiles, with IL-2 showing stronger response over IFN-gamma. There is a trend in favour of the lower dose administration to elicit an immune response, although the differences not significant. Although not powered to yield associations with functional immunity, this study is the first demonstration of immune responses in subjects using targeted antigen display on the self-assembling protein nanoparticle vaccine platform.
Vaseline Stefanova (University Health Network and, Toronto General Hospital, Canada) presented the outcomes of research on a diagnostic marker for severe malaria in children. Malaria is the leading cause of childhood mortality in sub-Saharan Africa, with host immunity and endothelial activation contributing to the pathogenesis of the parasite. However, early symptoms are similar among febrile children, and current malaria diagnostic tools do not reliably identify children whose symptoms are caused by malaria. However, results of the prospective study of febrile children identified soluble urokinase-plasminogen activator receptor (suPAR) as a prognostic marker in Ugandan children at risk of severe malaria. Elevated levels of this marker were identified in critically ill patients as evidence of disease progression and poor prognostic index. This marker can further improve the prognostic accuracy of a validated clinical scoring system and suggest earlier application of malaria- specific treatment. This approach could decrease morbidity and mortality of malaria in children under 5 across the endemic regions.
Wes Boland (Indiana University School of Medicine, United States of America) discussed the implications of perfusion index (PI) as an indicator and prognostic marker for mortality in children with severe malaria. Malaria remains the leading cause of death among children under 5 with severe malaria about 67% mortality. PI is recognized as evidence of peripheral perfusion and circulatory status, therefore a decline in peripheral perfusion is indicative of impending circulatory shock with corresponding outcomes. Boland’s study also found correlation between lower PI and the presence of subjective shock measures including capillary refill >sec, cold peripheries and lower limb temperature gradient. This finding suggests that PI could potentially be used as an alternative objective measure to diagnose shock in children with severe malaria. The findings also suggested the higher odds of in-hospital mortality with decreasing PI in cerebral malaria than severe malaria. These adverse outcomes are mostly seen in cerebral malaria cases. Therefore, efforts are being geared towards assessing the severity of malaria using pulse oximeter to measure the level of oxygen perfusion and assessment of shock.
Julie Wright (University of Toronto, Canada) presented the association of malaria in pregnancy (MIP) with intestinal permeability and preterm birth. Every year, 33 million pregnant women are at risk of malaria and the majority of these cases are in low-and middle-income countries. MIP is associated with adverse maternal and child outcomes including multi-organ failure, deaths and small for gestational age babies. The pathogenesis of this effect includes: sequestration of Plasmodium falciparum in the intestinal microvasculature; high grade parasitemia and bacteremia with corresponding metabolic acidosis observed in severe malaria as a result of the organic anions located within the gut. Similarly, severe malaria infection also induces low levels of bioavailable L- arginine resulting in increased gut leak which further worsens pregnancy outcomes. Wright’s study hypothesized that disruption of intestinal barrier integrity and gut leak induced by MIP is associated with these adverse pregnancy outcomes, and bioavailable L-arginine in MIP is inversely associated with gut leak. The findings from this study suggest that MIP is associated with intestinal disruption and gut leak (measured using 2 different markers i.e. CD14 and LBP) leading to deficiency of L-arginine. These factors all increased the odds of preterm birth.
Symposium #19: Malaria: Epidemiology I – Surveillance Strategies
Arnau Pujol (Barcelona Institute for Global Health – ISGlobal, Spain) presented a study assessing the potential of pregnant women to reflect spatial and temporal patterns of malaria transmission in the community. Pujol compared polymerase chain reaction positivity rate (PRPCR) from pregnant women from the MiPMon project with PRPCR from cross-sectional studies in children 2-10 years old. They also detected PRRDT for pregnant women by defining a detection threshold, whereas results from PRRDT for children were also available. They further evaluated the impact of different factors in pregnancy using subpopulations such as parity and HIV status. Both of these studies were conducted during the same time period in Manhica, Magude and Ilha Josina in Mozambique. The results showed strong correlation between PRPCR from pregnant women and children 2-10 years regardless of HIV status or parity in all transmission settings. On the other hand, for PRRDT, parity affected the linear regression slope i.e. lower with multigravid women and close to 1 for primigravid women. The study also revealed that temporal changes in PR from pregnant women were consistent with changes in incidence from health facility data with a time lag of 2 to 3 months.
Melissa Conrad (University of California, United States of America) discussed her study which showcased how school-aged children are the most important contributors to the human infectious reservoir for malaria in areas of high and low P. falciparum transmission intensity in Uganda. Children and adults were enrolled in cohorts in adjacent districts in eastern Uganda: Tororo district, where malaria transmission has diminished following effective vector control, and Busia district, where malaria transmission remains high. They quantified parasite carriage and contribution to transmission was measured via membrane feeding experiments in two settings of markedly different malaria transmission intensity in Uganda. Participants were recruited for passive and routine assessments. They found that despite marked differences in transmission intensity and clinical malaria incidence, children were responsible for most mosquito infection events in both settings. There was strong association between mosquito infection and gametocyte densities in both settings. They also found that asymptomatic infections were primarily the infection reservoir regardless of transmission intensity. She also mentioned that modeling work is ongoing to understand the contribution of age to the infection reservoir.
Christine Markwalter (Duke Global Health Institute, United States of America) discussed a study on malaria importation into an epidemic-prone setting in arid Northwest Kenya (Central Turkana). Here, P. falciparum events as well as its effect on local transmission was explored, and to achieve this, parasite collection was done in two groups – about 1,891 bus and plane passengers entering the region (active case detection) and from 1,891 RDT-positive index patients at 6 health facilities and 3,314 household members (re-active case detection). Subsequently, the parasites were genotyped using amplicon deep sequencing of pfcsp and pfama1, for which haplotypes were inferred using established methods. Results showed that P. falciparum qPCR positivity rate in inbound passengers was lower than in local household members. Overall, parasite importation by travelers into Central Turkana was detected; however, malaria is endemic and is sustained by local transmission. Therefore, interventions designed to suit the Turkana’s unique ecology would help to drive down transmission.
Lek Dysoley (National Center for Parasitology Entomology and Malaria Control, Cambodia) discussed the impact of primaquine on Plasmodium vivax relapse among patients in Cambodia. He explained that Glucose-6-Phosphate Dehydrogenase (G6PD) testing to inform safe treatment with primaquine has been recommended by WHO to facilitate the safe use of primaquine to prevent relapses from dormant liver-stage P. vivax parasites. He described a phase 1 trial evaluating the impact of using G6PD RDTs and 14-day primaquine (PQ14) treatment as a radical cure on local P. vivax transmission. They analysed P. vivax cases, before and during the radical cure pilot to determine if their introduction caused a reduction in P. vivax and relapse cases. In total, 31,175 P. vivax records were used in relapse analysis, evenly distributed between radical cure and non-radical cure provinces. Results showed that P. vivax cases continued to decrease from about 3068 cases nationwide and 426 (14%) in radical cure pilot provinces in January 2018 to 423 nationwide cases with 110 (26%) cases in radical cure provinces in December 2020. He concluded that radical cure has proven to be a valuable intervention in the ongoing P. vivax elimination efforts in Cambodia and the Greater Mekong sub-region, as there is evidence of reduced relapse rates since its introduction in the 4 pilot provinces.
Jessica McCaffery (Centers for Disease Control and Prevention – CDC, United States of America) presented the results of the deletions of P. falciparum histidine rich protein (HRP) 2 and 3 genes from persons presenting at health facilities in the Democratic Republic of the Congo (DRC), Ethiopia, Kenya, Madagascar and Rwanda. They screened individuals enrolled in therapeutic efficacy studies of antimalarial treatments with a bead-based multiplex assay detecting HRP2 and pan-Plasmodium lactate dehydrogenase (LDH) and aldolase. Samples with low HRP2 signal relative to LDH or aldolase were then genotyped for pfhrp2/3 deletions. They showed a sporadic and low prevalence of pfhrp2/3 deletions from DRC, Ethiopia, Madagascar and Rwanda with no deletions observed in Kenya, demonstrating that RDTs based on only pfhrp2 should still remain an effective testing strategy in these countries as the levels of pfhrp2 and/or pfhrp3 deletion exceeding 5% were not observed. McCaffery and her team recommend regular monitoring of the prevalence of pfhrp2/3 deletions to ensure RDTs remain reliable.
Philipp Schwabl (Harvard University, United States of America) started his talk by justifying the purpose of their surveillance in Guyana. He explained that low transmission environments like Guyana are conducive to drug resistance emergence and a new mutation (Kelch13 C580Y) was observed in the last decade in Guyana that is resistant to artemisinin. His team applied a new multiplexed amplicon sequencing protocol for rapid molecular surveillance to monitor the persistence and possible expansion of C580Y-carrying parasites in >1000 P. falciparum-infected blood samples collected from individuals in Guyana between 2010-2020. Furthermore, they intersected the amplicon sequencing results with 800+ whole-genome sequences from a 2016-2017 survey and found that various other clonal clusters occur between the two timepoints, suggesting that stochastic boom-bust dynamics can skew haplotype estimates inferred from cross-sectional sampling. Key takeaways were that amplicon multiplexes can cost-effectively extend/verify inference from whole-genome sequencing. Also, Kelch13 mutants are not expanding in Guyana like in Southeast Asia either because compensatory background is missing or broken apart by recombination. Transmission seemed not to be low/spatially restricted as to sustain clones with limited fitness advantage; and unstable genotype prevalence complicated cross-sectional interference. There are future plans to explore if they can reconcile observed rates of genetic change over 1.5 vs 4-year timescales with data on mixed infections and spatial gene flow/miner movement in Guyana.
Symposium #22: Malaria: Vaccines
RTS,S/AS01E has shown moderate vaccine efficacy when applied in African children using a scheme considering three primary doses at months (M) 0, 1 and 2, and then a delayed 4th dose given at M20; also, controlled human malaria infection studies in malaria naïve adults showed improved efficacy when a 3rd and/or 4th fractional dose was applied. With this context, Aaron Samuels (Centers for Disease Control and Prevention – CDC, Kenya) presented the results of the ongoing phase 2b open label randomized, controlled trial evaluating the efficacy of RTS,S/AS01E fractional dose (fx) regimens under conditions of natural exposure. Experimental regimens consisted of 2 full doses at M0 and M1 with either [i] full doses at M2 and M20 (R012-20), [ii] M2 and M14 (R012-14), [iii] Fx doses (1/5 of full dose) at M2 and M14 (Fx012-14), or [iv] at M7 and M20 (Fx017-20). 1500 Kenyan and Ghanaian children aged 5-17M were randomized (1:1:1:1:1) to receive RTS,S/AS01E (n=300/group) or a rabies control vaccine (n=300/group). All experimental regimens showed significant vaccine efficacy against the first episode of clinical malaria 12M post dose 3 and were well tolerated with no safety signals observed. Fx doses regimens did not show superior efficacy to full dose regimens; neither were they inferior. The vaccine efficacy obtained ranged from 35% (Fx012-14; 95%CI: 13-51%), to 47% (R012-14+R012-20; 95%CI: 31-59%), and 54% (Fx017-20; 95%CI: 38-66%). Trial is still ongoing as planned with continued follow-up until study end (M50).
From 2018, Hansenula polymorpha is used to produce R21, a construct with the circumsporozoite protein, fused to HBsAg. R21 is used with the Matrix-M™ adjuvant, a 40 nm lipidic complex, to form the R21/Matrix-M (R21/MM) malaria vaccine and Mehreen Datoo (University of Oxford, United Kingdom) presented its clinical development. R21/MM first showed high efficacy in phase I/IIa CHMI trials in naïve adults in the UK; a phase 1b age de-escalation trial in Kenya, showed good safety and immunogenicity in 91 adults, children and infants. A phase IIb field-efficacy study in 450 infants (5-17 months) in Burkina Faso administered three monthly doses prior to the malaria season. Efficacy of R21/MM with a high adjuvant dose for the first clinical malaria episode was 77%, while 76% for multiple episodes. A booster dose applied 12 months after first inoculation conducted to 81% and 77% efficacy for the first clinical episode and multiple episodes, respectively, after 24 months follow-up. R21/MM had a favourable safety profile and high NANP specific IgG levels were observed, which correlated with vaccine efficacy. The highest levels of NANP specific IgG appeared 28 days after 3rd dose, and booster dose restored them to peak quantities. A phase III double-blind, randomised controlled trial has started across four African sites to test R21/MM at differing transmission patterns and malaria burdens, in a broader age-range of children. First results are expected in 2022.
The excitement produced by the use of mRNA as a platform for infectious disease vaccines in humans has been renewed given recent approvals and showed efficacy. Evelina Angov (Walter Reed Army Institute of Research, United States of America) replacing Ishita Waghela, presented the potential of an mRNA malaria vaccine based on the circumsporozoite protein of Plasmodium falciparum (PfCSP). Immunogenic and protective potential of PfCSP mRNAL-NP was assessed using an array of dosing factors including formulation, dose, interval, and number of immunizations. Experiments were carried out using two different mRNA transcript sequence elements (TriLink/unmodified and UPenn/nucleoside-modified) and were delivered using LNP1 lipid nanoparticle structure in two mouse strains (BALB/c and C57BL/6). The in vitro inhibition of liver stage development assay (ILSDA) was used to test the inhibition of sporozoites. Both PfCSP mRNALNPs induced protective responses in mice. Low doses as 1?g induced durable antibody titers, and inhibitory antibodies produced by the UPenn/nucleoside-modified element were stable up to six weeks after final dose. Dose, schedule or mouse strain did not impact significatively the results. These findings call to consider the suitability of mRNA-LNPs for their use as vaccines in malaria.
Luna Barroco de Lacerda (Oswaldo Cruz Foundation Fiocruz-Minas, Brazil), discussed the designing of a malaria vaccine based on antigens presented by P. vivax (Pv)-infected reticulocytes. Many vaccines focus on the liver stage of the infection whose challenge is ability to activate antigen (Ag)-specific T cells or the blood stage of infection whose setback is that target antigen polymorphism requires high antibody titers. However, Barroco’s group observed that cytotoxic CD8+ T cells recognize and kill Pv-infected reticulocytes. Fifty peptides were tested and 3 recombinant proteins (L30, S25, H2A) produced. Mice were immunized three times with each protein together with an adjuvant, followed by challenging with P. yoelii (Py)-infected reticulocytes. Findings indicate that 23 out of 50 peptides were immunogenic during the acute infection but 12 peptides remain immunogenic in the convalescent phase, suggesting an immunological memory. Additionally, Pv Ag-specific CD8+ T cells are shown to kill Py-infected reticulocytes, suggesting a cross-species protection. Lastly, L30 and S25 proteins can not only induce antigen-specific IgG1 and IgG2c responses, but also protective responses that resulted in the inhibition of up to 50-80% the parasitemia compared to the control group. Therefore, new Pv antigens were found to induce a protective response to Py infection, indicating cross-species protection and a potential vaccine candidate.
Issaka Sagara (University of Sciences Techniques and Technologies of Bamako – USTTB, Mali) presented findings of a phase 2 field trial of Pfs230D1-EPA/AS01E, a malaria Transmission Blocking Vaccine (TBV), in Mali, West Africa. Following an age de-escalation pilot study for safety (in 9-18 year olds followed by 5-8 year olds), a community based trial enrolled extended families as a unit (referred to as a vaccine unit [VU]) who were then vaccinated collectively on 0, 28, 56 days schedule with either Pfs230D1-EPA/AS01E or comparator vaccine; children <5 years of age also were enrolled with their VU for parasitemia endpoints. All vaccinated individuals (5 years of age or older) were then followed post vaccination for safety, immunogenicity, and functional activity by standard membrane feeding assays (SMFA). School-aged children (9-18 year olds) underwent further assessment for functional activity by direct skin feeds (DSFs) every 2 weeks post the third vaccine dose for 8 DSFs and one year later, every 2 weeks post dose four for 10 DSFs with lab raised Anopheles coluzzi mosquitoes. No safety issues post vaccination or adverse events related to direct skin feeding (DSFs) were noted. Pfs230D1-EPA/AS01E showed an overall 73.6% efficacy against parasite transmission via DSF over two years combined; together justifying further studies as a viable malaria vaccine candidate alone or in combination with pre-erythrocytic vaccines.
Sara Healy (National Institute of Allergy and Infectious Diseases – NIAID, United States of America) in her talk emphasized the need to enhance protection against Plasmodium falciparum (Pf) infection in pregnant women and therefore reduce maternal, perinatal and infant mortality. Healy outlined successes of her group presented at previous meetings, including establishment of pregnancy registries at study sites as well as efficacy of 1-month PfSPZ Vaccine regimen and safety among women of childbearing potential. In a double-blind placebo controlled trial, Sanaria® PfSPZ Vaccine was administered to women of child bearing potential who planned to become pregnant during the study period but who also agreed to pregnancy prevention during vaccinations. The women were followed for approximately 1.5 years post enrollment for pregnancy. Pregnant women, and their offspring, underwent exploratory analysis for safety and parasitemia. Results indicated that recent receipt of PfSPZ Vaccine prior to pregnancy is safe and does not adversely impact pregnancy rate. Further, the study showed that PfSPZ Vaccine provides protection against parasitaemia in women who become pregnant within 6 months of vaccination. Future analyses will adjust for date of conception, IPTp dosing, prior parasitaemia, gravidity, seasonal variation of the parasite as well as immune responses against infection to fully elucidate durability of protection and fully characterize receipt of PfSPZ Vaccine in women who become pregnant shortly after vaccination and supports future studies in pregnant women.
Felicia Watson (University of Washington, United States of America) representing her group discussed considerations to translate the ‘Prime-and-Trap’ malaria vaccine strategy targeting the pre-erythrocytic stage of infection to the clinic. Prime-and-Trap entails the trapping of P. yoelii circumsporozoite (PyCSP)-specific CD8+ T cells elicited by DNA priming into the liver by giving a booster with liver-homing radiation attenuated sporozoite (RAS). Although Prime-and-Trap confers durable protection in a rodent malaria model, the requirement for high doses of IV administered RAS is a potential impediment for translation to larger animal models or humans. Therefore, this group analysed whether translational potential to clinic could be improved by reducing the RAS dose, eliminating IV RAS administration, and adding a glycolipid adjuvant, 7DW8-5. They observed that the efficacy of Prime-and-Trap with IV RAS administration is durable with or without the adjuvant for at least four months; co-administration of RAS+7DW8-5 confers protection in mice models with a 4-fold lower dose, and intradermal delivered RAS+7DW8-5 confers protection after Prime-and-Trap. In conclusion, Felicia stated that Prime-and-Trap is a promising and translatable vaccine strategy, co-administration with a glycolipid-adjuvant is dose sparing and improves efficacy with intradermal Py RAS delivery.
Symposium #27: Expanding the Use of Radical Cure to Patients with Uncomplicated Malaria Due to Any Plasmodia Species
Robert Commons (Menzies School of Health Research, Australia) began by reminding us that Plasmodium vivax (Pv) is now the predominant species of malaria in Asia and the Americas. He stressed the importance of improving the radical cure of P. vivax to kill hypnozoites, the silent reservoir of infection in a human host. He presented results from an individual patient pooled meta analysis defining the risk of Pv parasitemia after P. falciparum (Pf) infection. The biggest cause of recurrent malaria by day 63 was due to Pv rather than Pf, and this was particularly apparent after ACTs with short half-lives such as artemether-lumefantrine. The risk of Pv was also greater in areas of high relapse periodicity, in children, and in patients who cleared their initial parasite clearance slowly. The latter suggests that Pv reactivation might be triggered by symptomatic Pf malaria. In conclusion, in some co-endemic areas there is a high risk of Pv after Pf infection, suggesting potential benefit of treating all patients with uncomplicated malaria with ACT plus primaquine.
Jeanne Rini Poespoprodjo (Gadjah Mada University, Indonesia) presented a cluster randomised study of supervised versus unsupervised primaquine for the treatment of Pf or Pv malaria in Papua, Indonesia. In malaria endemic countries many patients do not complete the 14 day PQ recommended regimen and this leads to poor adherence and antirelapse effectiveness. In the Indonesia trial, 223 patients were randomised to a 14 day regimen of primaquine (total dose 7mg/kg), which was supervised on alternate days, and in the other arm, 196 patients received unsupervised primaquine. The clusters were selected according to location, size and annual parasite incidence. The results showed that at 6 months follow up, partial supervision of 14-days primaquine significantly reduced the risk of recurrent Pv by 77% and the rate by 37%. Importantly the benefit of partial supervision was apparent in patients presenting with Pf malaria, reducing Pv recurrence by 48%, supporting the idea that universal radical cure in areas with a high risk of Pv and Pf might work be implemented. Alternate day supervision may not be feasible so now studies are underway to find ways of improving adherence with fewer follow up visits.
James Walker (University of Melbourne, Australia) presented a malaria model to compare treatment for multispecies infections (A multispecies malaria model). The aim was to develop a model that could compare both Pv and Pf dynamics and the interactions between those in an individual host. This would allow for scenario modelling of different treatment strategies. His presentation focused on the universal radical cure scenario, using casework load data from Cambodia to validate his model. The scenario analysis showed that implementing a unified treatment policy of ACT plus 14 day primaquine for patients presenting with uncomplicated malaria due to any species, lead to significantly fewer infections and deaths in the population over a ten year period. James ended by suggesting that future work should be done in the form of sensitivity analysis, since many of the parameter estimates in the model may differ significantly in different endemic settings.
Angela Devine (University of Melbourne, Australia) introduced her talk on the cost-effectiveness of universal primaquine radical care policy by highlighting some of the benefits of universal radical cure, which included accurate diagnosis, and ensuring that all patients get effective treatment for the particular malaria species they are infected with. Angela reminded us that misdiagnosis of Pf malaria as Pv, resulted in results in patients being treated with chloroquine that could result in recurrent infection and even death. Pragmatic benefits of a universal approach include simplified patient flow for health workers and fewer drugs to keep in stock. Using data derived from the model that James Walker presented, she conducted an exploratory cost-effectiveness analysis for Indonesia, for implementation of ACT plus 14 days primaquine active against all l species of human malaria. In view of the risk of PQ induced haemolysis, all patients were tested for G6PD-deficiency before prescribing PQ, and this incurred additional costs. However, her analysis showed that universal radical cure policy can avert a large number of cases, at a cost of $112 per case of malaria averted. In a sensitivity analysis on G6PD test cost and the results showed potential to become cost-saving in high burden areas, decreasing the costs to $35 per case averted. In summary, a universal radical cure for malaria has many potential operational and economic benefits to reduce the burden of malaria.
Symposium #28: Early Experience and Next Steps in the Evaluation of the Attractive Targeted Sugar Bait for Malaria Control in Kenya, Mali and Zambia
Helen Jamet‘s (Bill & Melinda Gates Foundation – BMGF, United States of America) presentation gave us a detailed overview on the development of attractive targeted sugar baits (ATSB) and the research that is being done towards introducing ATSB as a new malaria prevention method. She explained that vector control tools must seamlessly integrate into daily life and that novel control methods must exploit mosquito behavioural patterns like ATSB does. ATSB exploits mosquito feeding behaviours by using a sugary syrup mixed with a toxicant to attract and upon ingestion, kill mosquitoes. Westham Co. developed an ATSB prototype whose proof-of-concept was observed in Mali in 2017. This became fundamental for data collation of product organisation and testing and entomological validation in Mali, Zambia and Kenya. The data obtained from this research will be used to demonstrate the public health value of ATSB. However, before public health value can be demonstrated, lab and field work are still critical to optimise product specifications, to determine product cost-effectiveness as well as ensuring WHO approval prior to ATSB’s commercial use. Jamet concluded her talk with enthusiasm towards the influential impact that ATSB will have on the vector control landscape if it becomes successful.
Eric Ochomo (Kenya Medical Research Institute – KEMRI, Kenya) stated that mosquito feeding rates on attractive sugar bait without toxin (ASB) established in Mali demonstrated the effects of ATSB on mosquito populations. From these research trials, it was found that daily feeding rates were similar between 2 and 3 ASB stations whilst a modelling frame suggested that a feeding rate of 2.5% is necessary to reduce malaria incidence by 30% in 2 years. This study, conducted in Zambia and Kenya, aimed to determine feeding rates of Anopheles funestus and A. gambiae and to assess the temporal distribution of feeding rates. The results showed that A. funestus feeding rates (in Kenya and Zambia) surpassed the 2.5% modelling threshold whilst A. gambiae’s feeding rates in Kenya did not surpass this threshold OR while the same did not hold true for A. gambiae’s feeding rate in Kenya. No significant difference was found in the number of ASB stations used in both countries suggesting that the same would apply to ATSB stations. A significant difference was observed in feeding rates between A. funestus and A. gambiae in both Kenya and Zambia with A. funestus, the primary vector, having a higher feeding rate than A. gambiae. The results imply that ATSB would be very effective against A. funestus, especially during the rainy season when increased feeding rates were observed. Increased feeding rates do not, however, affect the efficacy of ATSBs.
Kafula Silumbe (PATH, Zambia) stated that ATSB is a promising new vector control tool, however, community engagement (CE) is vital for its success. He conducted a randomized control efficacy trial to demonstrate ATSB’s potential public health importance via CE strategies. These aimed to support high intervention coverage and foster participation in trial study components that will form guidelines for future ATSB field trials. Challenges faced during the trial were widespread rejection of the intervention due to inadequate information or poor perception of ATSB by the local community. The importance of using CE strategies was highlighted during community meetings or consent and installation processes where ATSB knowledge was garnered by community members. The CE approaches implemented were ensuring a dedicated budget with project staff to develop, implement and monitor activities as well as training community members to lead sensitization efforts, retrieve and share community feedback routinely with the study team. Furthermore, health staff had to be engaged to readily handle severe adverse events. Silumbe emphasised the importance of having a feedback loop between community members and study liaisons to identify and rapidly resolve any concerns or questions and concluded that crisis communication plans are vital for guiding rapid responses to unanticipated events.
Mohamed Traore (University of Science and Technology of Bamako, Mali) presented findings from an entomological field study conducted in Northern Mali in 2019 to explore the potential of ATSB in a low-endemicity setting, with the primary vector being An. gambiae s.I. He explained that a pre-post study design was used, in which ten villages received 5,677 ATSBs (2 ATSBs per eligible structure) and another ten villages served as the control group with no ATSBs. The ATSB installation period was from July until September and stations were removed in late December of the same year. Entomological measurement was conducted monthly with mosquitoes being collected using CDC-UV traps, pyrethrum spray catch, and indoor and outdoor human landing catches. A mixed methods approach was used to process the mosquitoes. This included ovariole dissection and gonotrophic cycles quantification, ELISA to detect sporozoites and PCR for species identification. The result of comparison between ATSB intervention and control villages showed lower female An. gambiae s.I. abundance with lower proportions that were positive for malaria sporozoites (near zero) and with ≥ three gonotrophic cycles in the test group. These observations suggest that ATSBs have the potential to make a significant impact on malaria transmission in areas of low transmission.
Symposium #33: Towards a Next-Generation Malaria Vaccine Portfolio: Innovations in Malaria Vaccine Development
B. Kim Lee Sim (Sanaria Inc., United States of America) started her presentation sharing WHO’s hopes of ‘eventually having a vaccine that is 95% effective and can essentially eradicate malaria’. Then she highlighted the primary goal of Sanaria as to develop and commercialise a vaccine that is well tolerated and safe and can be used as a tool for malaria elimination. They have developed Plasmodium falciparum (Pf) sporozoite (SPZ) vaccines using aseptic, purified, live, attenuated vialed cryopreserved PfSPZ that meet regulatory standards. She discussed the Pf development in the liver and the importance of PfSPZ vaccines on the prevention of homologous and heterologous controlled human malaria infection (CHMI) with Pf parasites. In order to achieve their aim, Sanaria is focusing on four major research areas which include: (i) to receive marketing authorization and initiate commercialization for the radiation-attenuated PfSPZ vaccine, (ii) to reduce costs of goods by 75% to 80% by increasing potency, (iii) to reduce costs of goods by 80% to 90% and further increase consistency and reproducibility of manufacturing, and (iv) to use a PfSPZ vaccine as the lead agent in malaria elimination campaigns, currently focusing on supporting Equatorial Guinea and Tanzania. She explained their evidence-driven strategies to achieve these plans, as well as ongoing and planned clinical trials. She mentioned that the first PfSPZ vaccine for Pf malaria prophylaxis will move to marketing authorization in 2023. Additionally, their discovery of a hollow fiber culture system for mass production of in vitro PfSPZ will reduce the cost of producing vaccines.
Simon Draper (University of Oxford, United Kingdom) commenced by presenting on the challenges facing development of blood stage malaria vaccines which includes (i) antigenic polymorphism and redundant invasion pathways of leading antigen targets; (ii) lack of in vitro correlate of in vivo protection; and (iii) the need to induce an extremely high antibody concentration for protection, and to maintain this for a useful duration of immunity. He discussed extensively studies that highlighted the rapid progress made on the development of next-generation blood-stage vaccines for both Pf and Plasmodium vivax (Pv). This progress has been enabled by improved essential or conserved target antigens, improved tools to measure antibody structure-function relationships, and optimized delivery (quantity, quality and longevity) of vaccine-induced antibody responses.
Hernando del Portillo (Barcelona Institute for Global Health & Germans Trias i Pujol Research Institute, Spain) discussed Plasmodium vivax infection, which accounts for 2.5 billion people at risk and up to 7.5 million clinical cases. The parasite is endemic in Latin America and Asia. P. vivax is resistant to elimination – about 70% of infections are asymptomatic and poses a challenge for control of infection when compared to P. falciparum. More so, recent clinical data suggest that P. vivax causes cryptic erythrocytic infections with spleen and bone marrow invasion, different from P. falciparum infection which is mostly in the circulatory peripheral blood. The pathogenesis makes the elimination of P. vivax more demanding, and P. vivax cannot be controlled or eliminated if we depend on the present tools being used to combat P. falciparum infection. Therefore, we need new tools such as the novel discovery of reticulocyte-derived exosomes as a new antigen for vaccine development against P. vivax malaria.
Ashley Birkett (PATH, United States of America) presented lessons learnt from the Covid-19 pandemic to inform the development of malaria vaccines. This was premised on sharing contrasting views with respect to research and development (R&D) and investment opportunities between Covid-19 vaccine and the new malaria vaccine. Although both infections are of global health emergency, the process of development of the Covid-19 vaccine was facilitated within a year compared to the WHO approved RTS,S malaria vaccine that took years. The biological differences posed by malaria, long follow-up time, and funding gaps were some of the challenges identified. However, the Covid-19 vaccine received $5.5 billion funding support for R&D within the first 12 months, while all malaria R&D had an annual investment of $600-700 million, of which the vaccine R&D had a share of about $130 million. Additional support of $600 was received from partners and countries for scale-up of Covid-19 vaccine; being some examples of the game change witnessed in the Covid-19 vaccine production. Similarly, new malaria R&D opportunities such as the mRNA vaccine and investment in African manufacturing companies were discussed.
Symposium #35: Community Delivery of Intermittent Preventive Treatment (IPTp): How It Contributes to the Goal of Improved Maternal and Newborn Health Outcomes
Introductory remarks by Pedro L. Alonso (WHO Global Malaria Programme, Switzerland) emphasized the importance of preventing malaria in pregnant women so as to eliminate adverse events associated with malaria in pregnancy such as maternal mortality, anaemia, low birth weight, and infant mortality. He pointed out that tools against malaria prevention are not perfect alone and there should also be concerted efforts in timely testing, diagnosis and treatment of malaria. Alonso further emphasised that antenatal clinic (ANC) visits are crucial to reducing missed opportunities for diagnosis and treatment. He lauded the steady increase in overall IPTp coverage from 2% to 34% over the last 10 years but noted the unacceptably low coverage in pregnant women. Alonso in conclusion encouraged development of projects which explore community-based approaches to complement existing measures to increase IPTp access; making a call to action to protect pregnant women.
Odete Cossa (Jhpiego, Mozambique) introduced the 5 year Transforming Intermittent Preventive Treatment for Optimal Pregnancy (TIPTOP) project rolled out in Mozambique, Democratic Republic of Congo (DRC), Nigeria and Madagascar with the aim to reduce maternal and neonatal mortality by expanding access to quality-assured sulfadoxine-pyrimethamine (SP) for IPTp. TIPTOP has seen increased uptake of three doses of IPTp without decreasing antenatal clinic (ANC) attendance by achieving three goals. First, implementing community based IPTp-SP by ensuring strong partnerships with stakeholders; strengthening existing health systems; capacity building of health care providers, maintaining engaged communities; deploying trained community health workers (CHWs) to screen pregnant women and provide SP by directly observed therapy (DOT); and generating evidence to inform policy decisions. Second, improving supply of quality-assured SP. And third, by establishing support for transition, scale-up and sustainability of community delivery IPTp-SP (C-IPTp-SP) by the ministries of health in the four countries. Cossa noted that like many health projects, TIPTOP was impacted by COVID-19, however they sustained community essential services, and ensured protection of health workers and clients. Then she emphasized that success has to be grounded on engagement with community civil organisations and community leaders through government-led partnerships. She concluded by stating that community engagement ensures creativity, innovation and adaptability, all key elements for short and long term gains for prevention of malaria in pregnancy.
Herbert Onuoha (Jhpiego, Nigeria), working with the TIPTOP project also emphasized that robust health systems management information (HMIS) and engagement of Ministries of Health (MOH) are keys to successful project implementation. Rapid facility assessments (RFAs) revealed varied gaps across all the countries related to the HMIS system and its utilization that was tackled by using a targeted approach. New data elements were added in HMIS for IPTp reporting and C-IPTp was integrated into an existing data flow. Stakeholder-led partnerships were key to ensure coordination, sharing and planning within TIPTOP, which also employed mobile data collection and reporting for C-IPTp data which ensured timeliness. However, some challenges such as increased requirements for smart-phones and data connectivity were observed. Onuoha concluded by pointing out that engaging MOH and other stakeholders ensured effective C-IPTp implementation and monitoring; strengthening existing HMIS to include data from private sectors and the community drove programmatic and technical quality for sustainability, and finally that regular supportive supervision and positive feedback motivates service providers for improved service and data quality.
Ogonna Nwankwo (University of Calabar, Nigeria) presented the preliminary findings from a qualitative study that assessed the acceptability of C-IPTp and barriers and opportunities for C-IPTp uptake and antenatal care (ANC) attendance in the TIPTOP implemented countries. Overall, C-IPTp has been widely accepted by its beneficiaries in all project areas. However, community health workers’ (CHWs) workload and lack of incentives were identified as a barrier to C-IPTp. Health seeking barriers identified were perceived fear of sulfadoxine-pyrimethamine (SP) side effects, women’s lack of autonomy, and impaired access to health facilities due to financial and logistical constraints. Likewise, health-seeking facilitators identified by this study include trust in SP efficacy, awareness of malaria symptoms and severity, general acceptance of medical pluralism, involvement of relevant community stakeholders in dissemination of information, trust and acceptability of CHW and awareness of SP and ANC as important preventive measures. Ogonna concluded on the importance of addressing beneficiaries and CHWs concerns over C-IPTp delivery to reduce maternal and neonatal mortality due to malaria. He further emphasized replanning sensitization strategies that can address context-specific barriers.
Clara Menéndez (Institute of Global Health in Barcelona – ISGlobal, Spain) presented preliminary results from household surveys on key indicators including coverage of at least 3 courses of IPTp (IPTp 3+) and attendance to antenatal care clinics from 2018 to 2019/20. IPTp3+ coverage was much higher after one year of TIPTOP project implementation than at baseline for DRC, Nigeria and Madagascar, except Mozambique where the coverage decreased. Four those 3 countries the average number of courses per woman is more than 3. Attendance in ANC4+, ANC1+ or early ANC attendance remained stable or decreased during the period. Menéndez then presented an estimation of the effect of community IPTp (C-IPTp) on IPTP 3+ coverage using a difference-in-differences (DiD) method. This method allows to compare over time before-after changes in the intervention population with the before-after changes in a control population and to adjust for potential confounders. It shows that C-IPTp may have contributed to the increase in IPTp3+ coverage in all countries but Madagascar. Contextual factors such as dramatic meteorological events, insecurity and political changes as well as COVID-19 restrictions affected negatively both ANC attendance and IPTp3+ coverage in Mozambique.
Symposium #43: Clinical Development of Malaria Transmission Blocking Vaccines: Which Road Do We Take?
* Not yet available.
This report is brought to you by the MESA Correspondents. Senior editorial support has been facilitated by the Organizers and Co-Chairs of the symposia and Divya Beri (Lindsley F. Kimball Research Institute, New York Blood Center, USA). This report is cross-posted on the MESA website and on MalariaWorld.
Published: 18/11/2021
This report is brought to you by the MESA Correspondents Edima Ottoho, Tope Kayode, Franklin Tembongshu Formilack, Lucy W. Mwangi, Vita Mithi, Ana Alonso, Faith Hungwe, Olajoju Temidayo Soniran, Isabelle Delrieu, Patricia Doumbe Belisse, and Carlos A. Fernández Miñope. Senior editorial support has been facilitated by the Organizers and Co-Chairs of the symposia and Divya Beri.
THEMES: Basic Science | Epidemiology | Health Systems