ASTMH 2018: Session 30 “A Roadmap for Ivermectin as a Complementary Vector Control Tool for Malaria”
Published: 18/12/2018
THEMES: THEMES: Drug-based Strategies | Residual Transmission | Vector Control
MESA Correspondents bring you cutting-edge coverage from the 67th ASTMH Annual Meeting
Session 30: “A Roadmap for Ivermectin as a Complementary Vector Control Tool for Malaria “
Ivermectin is a licensed drug with an excellent safety profile that has been used widely against human onchocerciasis, lymphatic filariasis and other Neglected Tropical Diseases (NTDs) through intermittent, single dose, Mass Drug Administration (MDA) campaigns, and is also used for treatment of helminths in cattle. The recognition that mosquitos that feed on humans and other mammals treated with ivermectin suffer both direct (death) and indirect (changes in behaviour) effects has led to increased interest in the potential use of ivermectin as an adjunct vector control tool for malaria, particularly in the context of residual transmission. A Preferred Product Characteristics document (PPC) was published by WHO, and Ivermectin is now included in the range of priority Target Product Profiles (TPPs) for the not-for-profit public private partnership “Medicines for Malaria Venture” (MMV) as TCP6.
Unfortunately, the malaria community still lacks clarity on critical issues related to the development pathway, particularly the dose and drug regimen, and the specific studies required to guide the regulatory processes and policy pathway that would lead to licensure and effective use of ivermectin as a complementary vector control tool to reduce malaria transmission. A small number of ongoing trials with varying designs, regimens, endpoints, and sources of funding will provide some further data. Should ivermectin meet all milestones, the pathway to financing needs to be established for the malaria indication, since it is currently donated for Mass Drug Administration for Neglected Tropical Diseases. In addition, clarification of the clinical and regulatory pathway could facilitate development of novel candidates that could offer superior performance such as a longer half-life or requirement for less frequent dosing.
ISGlobal is currently leading a process to bring the community together to develop the Ivermectin Roadmap to development as a novel tool for the malaria community. The Roadmap reflects the work of 35 experts in their fields, covering clinical trials, entomology, drug development, vector tool development, malaria and veterinary medicine, ethics, industry, social scientists, programme implementers, past NMCP directors, NTD specialists, and modellers. The preliminary results of the roadmap process were presented in this symposium.
Presentation: Ivermectin Roadmap: efficacy and safety workstreams implications for the regulatory pathway
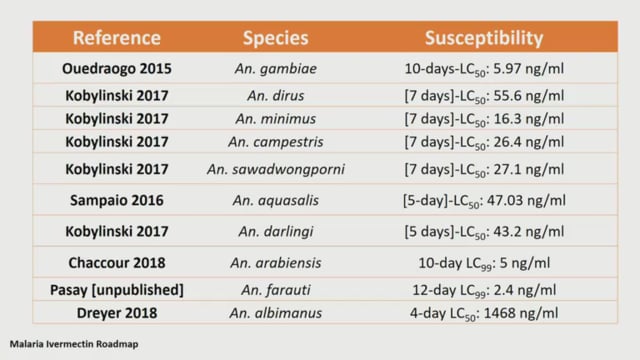
Carlos Chaccour (ISGlobal, Spain) provided a general overview of the roadmap process and the implications of the outputs of efficacy and safety workstreams for the regulatory pathway. He described the scenarios contemplated for use of ivermectin in the control-elimination continuum and used practical examples from ongoing or imminent trials to show the advantages and disadvantages of different doses and regimens.
Presentation: Ivermectin Roadmap: enhancing impact from One Health strategies
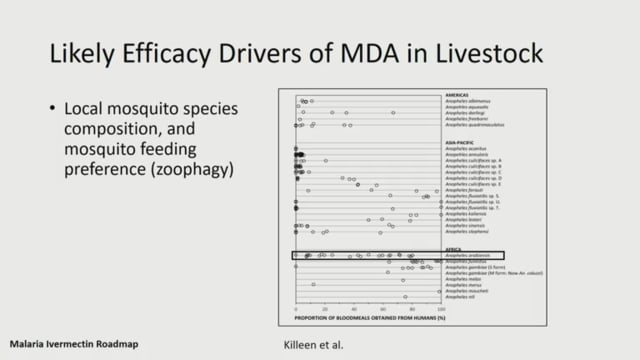
Cassidy Rist (Virginia Tech, USA) provided a general overview of the potential livestock application of ivermectin including: challenges regarding the target species, food safety and specific regulation. She highlighted the potential indirect economic benefits via increased yield, and discussed potential resistance in parasites of veterinary importance. She also discussed the need for assessment of the effects of mass cattle treatment on the environment.
Presentation: Ivermectin Roadmap: aligning with NTDs
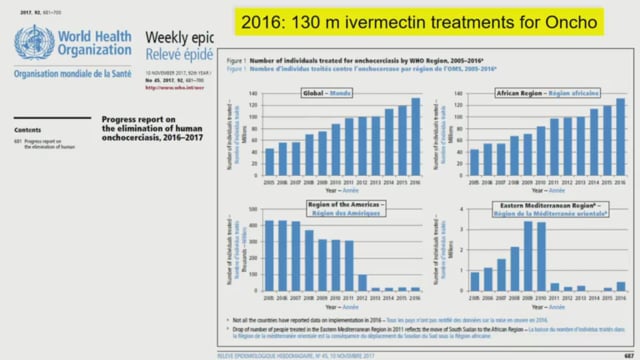
Frank O. Richards (Carter Center, USA) reviewed the lessons learned from NTD programs in the last 30 years. He discussed the expansion of ivermectin MDA for NTDs including manufacturing issues, and reviewed the challenges of drug distribution as well as the potential schemes used so far. He highlighted exit strategies and criteria for stopping MDA.
He finished by mentioning emerging drug combinations, touching on potential resistance in filariae in Ghana. His main conclusion was that multiple ivermectin treatments per year (for malaria) can help accelerate elimination of NTDs.
Presentation: Ivermectin Roadmap: Supply, Policy and financing opportunities
Jessica Rockwood (International Public Health Advisors, USA) gave an overview of the process for scale-up of interventions. She described the review processes of the WHO Vector Control Advisory Group (VCAG) and key steps regarding supply, namely (a) making the case for industry investment, (b) assessing WHO Pre-qualification requirements from potential suppliers and (c) requirements for donor financing. She provided the audience with a landscape of donors financing vector control commodities and finished with an approximate timeline for ivermectin implementation.
Chairs: Regina Rabinovich (ISglobal / Harvard) and Fred Binka (University of Health and Allied Sciences)
This report was written by Carlos Chaccour with editorial support from Professor Graham Brown.
Published: 18/12/2018
THEMES: Drug-based Strategies | Residual Transmission | Vector Control