1st Women in Malaria (WiM) Virtual Conference – 2021: Day 1
Monday, 22nd March 2021
Published: 22/03/2021
This report is brought to you by the MESA Correspondents Sushma Ambekar, Christine Markwalter, Rosheen Mthawanji, and Diane Leslie Nkahe. Senior editorial support has been facilitated by Joanne Power, Elena Gómez Díaz and Sarah Reece.
THEMES: THEMES: Basic Science | Epidemiology | Health Systems
MESA Correspondents bring you cutting-edge coverage from the 1st Women in Malaria Conference
Day 1: Monday, 22nd March 2021
Welcome and introduction
The first Women in Malaria (WiM) Conference (22 – 24 March, 2021) began virtually, with over 500 registrants attending from all over the world. The organizers, Elena Gómez-Díaz (Institute of Parasitology and Biomedicine Lopez-Neyra – IPBLN, Spain) and Sarah Reece (The University of Edinburgh, UK) began by welcoming everyone and providing a brief overview of the history, inspirations, and goals of the WiM community. WiM was created in 2018 to address inequities that women in Global Health and Parasitology (with a focus on malaria) face in their daily lives and within the scientific community. The goal of this conference is to showcase the contributions of women to the malaria field, to guarantee diversity and inclusion of all individuals, and provide training, mentoring and networking opportunities for women in the malaria research community. The entirely virtual format enables women from all around the world to participate in the conference.
Session 1 – New frontiers in “omics” approaches
The chairs, Angela Early (Harvard T.H. Chan School of Global Health, USA) and Victoria Ingham (Heidelberg University Hospital, Germany), welcomed participants to one of the two first parallel sessions of the conference, “New frontiers in “omics” approaches”, in which researchers use genomics, proteomics, and metabolomics screening techniques to get insights into the biology of the parasite and further the fight against malaria.
Silvia Kariuki (KEMRI-Wellcome Trust Research Programme, Kenya) discussed her novel approach to functional validation of human host variants that confer protection against clinical malaria. Focusing on the Dantu blood group variant, she performed in vitro invasion studies in red blood cells (RBCs) from homozygous, heterozygous, and non-Dantu individuals. Flow cytometry experiments identified a significant reduction in invasion in the Dantu homozygote RBCs across multiple P. falciparum strains, and live-video microscopy observation confirmed that merozoites attempted to invade Dantu RBCs, deforming the cells, but were unable to invade at the same rates as non-Dantu variants. Further biophysical characterization of RBCs across all blood groups identified RBC membrane tension as the primary mechanism by which merozoites were prevented from invading RBCs, and the median tension of Dantu-variant cells was significantly higher than that of non-Dantu cells. Kariuki’s work established a protective mechanism for Dantu variants whereby the inhibition of P. falciparum invasion is mediated primarily by its impact on RBC membrane tension. She noted that recent whole-genome sequencing of underrepresented groups has enabled improved host genome-wide association studies for novel host genes that confer malaria protection in African populations, and in the future, she plans to probe whether tension-mediated invasion inhibition is generalizable across multiple host variants associated with malaria protection.

Franziska Hentzschel’s (Wellcome Centre for Integrative Parasitology, UK and Heidelberg University, Germany) presentation was a comprehensive characterization of Plasmodium berghei across erythropoietic niches. Using mouse infection models, flow cytometry, and single-cell RNA sequencing (scRNA-seq), Hentzschel explored the distribution and transcriptomes of P. berghei parasites in different host cell types and host organ environments. Overall, parasitemia was higher in the spleen and blood compared to bone marrow, particularly for reticulocytes. Parasite scRNA-seq did not show any transcriptional differences between parasites in distinct erythropoietic organs. Despite an enrichment of infected splenic cells relative to blood, the vast majority of merozoite invasion occurred in circulating blood. Parasites demonstrated a bimodal invasion pattern with respect to host cell age, invading either early CD71+ reticulocytes or mature RBCs. Parasite transcriptomes differed depending on whether they invaded early reticulocytes or mature RBCs. Three genes associated with the purine salvage pathway were upregulated in parasites that invaded mature RBCs, which may be a response to decreased metabolite availability in mature cells compared to reticulocytes. A transcriptional factor for gametocyte commitment, AP2-G, was upregulated in parasites that invaded CD71+ reticulocytes, and in vivo studies confirmed a higher gametocyte rate in reticulocytes independent of erythropoietic organ. Overall, these data suggest that host cell age, rather than the host organ, determines transcriptional changes in the Plasmodium berghei rodent malaria model.
Lisl Esherick (Massachusetts Institute of Technology – MIT, USA) presented her work on developing a scalable pipeline for functional genetic analysis in Plasmodium falciparum. The CRISPR-based platform enables gene disruption via knockout and/or the TetR-DOZI system, which allows for conditional gene knockdown. In the latter approach, the sequence for a TetR-DOZI-binding aptamer is integrated into the genome upstream of a gene of interest. When TetR-DOZI is bound to the transcription product, translation is inhibited, resulting in gene knockdown. Adding anhydrotetracycline (aTc), which binds TetR-DOZI with high affinity, disrupts the complex and enables gene expression. Importantly, such an approach opens the door for conditional knockdown of essential genes. Using this platform, Esherick performed functional genetic analysis of a set of 60 pilot target genes with unknown function to determine their essentiality and potential as drug targets. Growth assays after conditional knockdown enabled the classification of targets as essential, slowed growth, or dispensable. Esherick applied this conditional knockdown system to drug target identification, demonstrating proof-of-concept with aminoacyl tRNA synthetases (aaRSs). 275 potential phenylalanyl-tRNA synthetase (FRS) inhibitors were screened and 13 hits were identified. In total, three inhibitors that targeted aaRSs were identified using this scalable CRISPR-based platform. In future, Escherik aims to use this new scalable pipeline for chemogenetic screening of pooled transgenic parasites (~250 genes) against small molecules of unknown function.
Carla Proietti (Australian Institute of Tropical Health & Medicine, James Cook University, Australia) discussed a systems-based approach for identifying novel antibody and T-cell targets for rational malaria vaccine and immunodiagnostic design. Protein microarrays populated with 2320 antigen fragments (representing ~25% of the proteome) were used to screen Plasmodium falciparum-specific antibody responses in serum samples. A highly multiplexed interferon gamma (IFN-γ) Elispot assay was used to probe T-cell responses to 29000 peptide epitopes from 1450 pre-erythrocytic antigens. Immune responses in a cohort of malaria-exposed individuals in Papua New Guinea demonstrated that only 30% of the parasite proteome is targeted by host immune responses, and both antibody and T-cell targets are dispersed throughout the genome. Additionally, T-cells and antibodies recognized distinct antigens, which could be accurately predicted (100% sensitivity and 70% specificity) based on 8 genomic attributes. These findings suggest that different vaccination approaches and antigen targets may be necessary, depending on the desired immune response. Building on this, a 1-year longitudinal study in Ghanian children showed an inverse relationship between the maximum intensity and breadth of IgG antibody responses, suggesting that next-generation malaria vaccines will need to target a combination of a large number of antigens to elicit a high-breadth response. Machine learning and regression techniques revealed a signature of IgG responses to 15 antigens that were predictive of clinical malaria immunity, and these signatures were validated in a separate Malian cohort of children. These findings could be useful in identifying novel vaccine or immunodiagnostic targets.
Mara Lawniczack (Wellcome Sanger Institute, UK) discussed two exciting projects in her group: (1) a novel targeted amplicon sequencing panel for Anopheles vectors and (2) the Malaria Cell Atlas. For the Anopheles project, the group developed a multilocus DNA barcode panel targeting 62 mosquito primer sites and 2 Plasmodium loci that enables a global view on Anopheles species present, their population structure, and if each contributes to malaria transmission. After a simple, cost-effective, and minimally-destructive DNA extraction, targets are amplified and sequenced. This approach is useful because it does not require prior knowledge of vector populations and can provide insight into changes in population structure after vector control interventions. It is applicable to any Anopheles sample, including adults, larvae, bulk traps, or environmental DNA in breeding sites. An in silico analysis showed within-species geographic population structure based on SNPs on 62 loci, and a 2-stage hierarchical classifier accurately (98%) assigned species to 133 wild-caught and 28 mosquitoes in silico. The second part of the talk focused on the Malaria Cell Atlas project which consisted in profilling single-cell transcriptomes of parasites in all stages in human and mosquito hosts for Plasmodium berghei and P. falciparum. This platform has been applied to answer a variety of research questions, and interactive data are available at malariacellatlas.org. Future work will look at the relationship between sexual/asexual parasite stages within single symptomatic and asymptomatic hosts in Mali, as well as building the Malaria Cell Atlas database for P. ovale and P. malariae.

Session 2 – Evolutionary ecology of malaria parasites
Farah Ishtiaq (Tata Institute for Genetics and Society, India) and Lindsey Plenderleith (The University of Edinburgh, UK) welcomed everyone to the second Women in Malaria conference session of the day, a session that focused on studying malaria parasites in the light of genetic and environmental factors.
Petra Schneider (The University of Edinburgh, UK) started her talk by highlighting the importance of understanding the evolutionary ecology of malaria parasites. Her work focused on the proportion of asexual malaria parasites that commit to producing thee gametocyte stage, i.e. the “conversion rate” of parasites into sexual forms. Schneider and colleagues collected data from Plasmodium chabaudi-infected mice that were exposed to different dosages of the antimalarial drug, Pyrimethamine, over time. It was observed that the parasites altered conversion rate (using phenotypic plasticity) according to the proportion of asexuals stages killed by each dose of pyrimethamine. Further, it appeared that Plasmodium parasites detected changes in their environment within the host by measuring changes in asexual parasite population density, as well as detecting changes in the number of host red blood cells. The results of this study demonstrated the predicted non-linear, fitness maximising (adaptive) reproductive strategy of Plasmodium spp. parasites, i.e. that smaller losses in asexual parasite density within the host blood resulted in a reduced conversion rate (reproductive restraint), with loss of an irrecoverable proportion of asexually-replicating parasites inducing an increased conversion rate (terminal investment). Schneider ended her talk by highlighting that an understanding of the mechanisms that underlie this adaptive plasticity in gametocyte conversion rate could inform future malaria control strategies as knowledge of these parasite behaviours can be used to manipulate the parasite into responding in ways that are detrimental to parasite fitness.

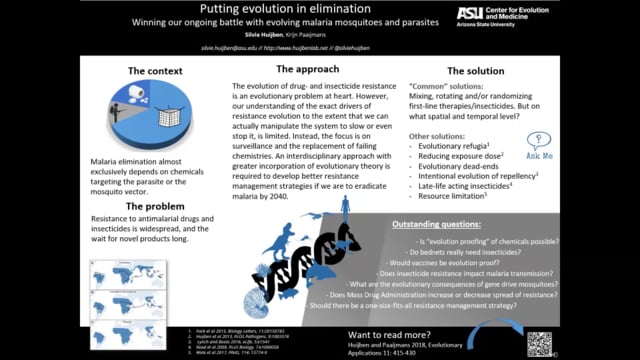
Silvie Huijben (Arizona State University, USA) presented her work on the evolution of both insecticide resistance and drug resistance and emphasized the global challenges of these issues in malaria control. The main questions that the Huijben group are trying to answer are if there are better ways to manage resistance and if resistance can be predicted. The evolution of resistance is sparked by two processes: mutations and the natural selection of the mutated entities. The current method used in treating resistance dates back to Alexander Fleming and is known as an aggressive treatment, where the dosage of the drug is increased to kill resistant populations of parasite or mosquito. In susceptible populations, natural fluctuations in the density will occur, however, the introduction of drugs leads to a population decline. Huijben used a rodent malaria model to show that if a resistant genotype exists in the population at the point when aggressive drug treatment is administered, then the resistant genotype will thrive and increase due to a release from competition as the susceptible strain reduces. Huijben suggests (1) minimizing chemical use, (2) evolutionary refugia, (3) fluctuating environments and (4) harnessing immunity as ways to reduce the spread of resistance. She ended her talk by introducing a mathematical model to determine which of the 4 previous suggestions is more efficient for drug/insecticide resistance management by exposing mosquitoes to different doses of an insecticide and determining selection.
Anne Vardo-Zalik (The Pennsylvania State University, USA) presented her findings on the effect of drought-like conditions on parasite ecology in the lizard malaria parasite, Plasmodium mexicanum, and the infection prevalence on its host, the western fence lizard, Sceloporus occidentalis. There is a good amount of research on how long-term drought conditions and vector-borne diseases intersect in terms of their factors like prevalence, transmission foci, and host concentration, but not on how such conditions affect competition or cooperation in multi-clonal infections. The Vardo-Zalik group collected data from California’s ‘Thousand-year drought’ in 2013-2014 and other droughts that occurred between 1978 – 2016 to look at parasite prevalence and genetic diversity. Accounting for variation in sex, size, and site of capture for each lizard, their analysis revealed that there was a significant decrease in clonality with drought-like conditions. Infection prevalence, however, showed contrasting results for short-term and long term data-sets. Regardless of the drought measure, there was always a positive association for the former and a negative association for the latter. Vardo-Zalik concluded her talk by saying that the disconnect between prevalence and infection complexity could be due to the transmission of multiple clones, and competition caused due to the drought or transmission foci.
Kathryn E Tiedje (University of Melbourne, Australia) started her presentation by throwing light on the importance of malaria disease surveillance using molecular and genetic approaches. Specifically, the work presented was focused on markers under immune selection such as variant surface antigen encoding genes. PfEMP1, encoded by the var multigene family, is the major variant surface antigen present on infected erythrocytes which frequently undergoes clonal antigenic variation allowing the parasite to successfully evade the host immune system. Var genes have previously been disregarded as biomarkers for malaria surveillance due to their diversity, but Tiedje and colleagues show that a 450 bp DBLɑ domain can be used to study Plasmodium global population structure at different spatial scales. They utilized the Jumping Hidden Markov Model (JHMM), taking consideration of recombination within var genes and investigated the relationship between DBLɑ types from Plasmodium laboratory strains and those isolated from chimpanzees and gorillas, showing the effectiveness of such an approach. They then applied the same approach to 1248 P. falciparum isolates from 10 countries. While the global population structure of DBLɑ types was clustered, indicating a distinct division across continents, the group noted the occurrence of the 100 most frequent DBLɑ types occurring globally, thus stressing the importance of elucidating their biological functions.
Ana Rivero (French National Centre for Scientific Research, France) began her talk by posing a question to the audience: “Are there any general laws in parasite ecology to explain recurring patterns?” This question was discussed within the context of how parasites in diverse hosts occur in quantifiable distributions, i.e. the majority of hosts have few parasites, but a small fraction of them have a high number of parasites. Aggregation/overdispersion of parasites has very important ecological and epidemiological consequences, but the underlying mechanism remains to be dissected. To this end, Rivero discussed the hypothesis that adaptive parasite plasticity leads to temporal heterogeneity. To test this, Rivero and colleagues explored whether the aggregated distribution of three strains of the avian malaria parasite, P. relictum, within Culex pipiens mosquitoes was explained by temporal heterogeneity in parasite burden and/or infectivity triggered by the bites of mosquitoes (with the Atlantic canary (Serinus canaria) as the avian host). An increase in parasitemia was observed in birds that were stimulated by mosquito bites for early and late chronic infections. When the group also observed a proportional increase in oocyst numbers for these infection stages in the mosquito, they set out to compare the number of oocysts in early and late biting mosquitoes (individual and batch) over a three-hour time period. The results of these experiments echoed some of their previous observations, showing higher oocyst numbers in later biting mosquitoes, despite no changes in the density of parasites within the host, suggesting that parasites become more infective in response to the biting of mosquitoes.
Workshop I
In between sessions, there was the first Workshop of the conference for pre-selected participants on Leadership.
Session 3 – Host-Parasite Interactions
The chairs, Maria Bernabeu (European Molecular Biology Laboratory – EMBL, Spain) and Camila Coelho (National Institutes of Health – NIH, USA), welcomed participants to the third session of the Women in Malaria Conference “Host-Parasite Interactions”, a session in which presenters explored the roles of human host factors on malaria infection.
Elizabeth Egan (Stanford University School of Medicine, USA) presented her work on establishing the identities and functions of red blood cell (RBC) factors in malaria parasite invasion. This is a particularly challenging line of work, because RBCs lack nuclei, making genetic studies less straightforward. Egan’s solution relies on genetically modifying human hematopoietic stem cells using CRISPR/Cas9 and subsequent ex vivo erythropoiesis. Cultured and genetically modified RBCs can be then infected with Plasmodium falciparum to systematically screen for novel host factors. Using this methodology, Egan’s group identified CD44 as a candidate critical for P. falciparum invasion. Subsequent assays showed that CD44 binds the erythrocyte-binding antigens (EBAs) 140 and 175, known targets involved in RBC invasion. Although the CD44-null RBCs did not show decreased EBA-175 binding, they did demonstrate lower surface phosphorylation relative to the wild-type, suggesting that EBA-175 induced phosphorylation of erythrocyte cytoskeleton proteins could be dependent on CD44. These findings on CD44 and future implementation of this workflow on other potential RBC factors could lead to novel strategies for anti-malarial interventions.
Ekta Saini (International Centre for Genetic Engineering and Biotechnology – ICEGB, India) shared with the audience a recent study on P. falciparum Photosensitized INA-Labelled protein 1 (PhIL-1) and the discovery of a novel PhIL-1 associated protein complex followed by its functional characterization. PhIL-1 is a part of the inner membrane complex (IMC) in the parasite and is associated with the parasite’s glideosome machinery. The proteins of this complex are required for propelling merozoites into the RBC during the invasion process. Microscopy studies conducted in collaboration with Rita Tewari (The University of Nottingham, UK) showed localization of PhIL-1 to the parasite IMC. Knockdown studies for PhIL-1 showed a reduction in various parasite life stages, but invasion remained unaffected. PhIL-1 was also seen to extensively interact with a glideosome associated protein (PfGAPM2), an IMC structural protein, Alveolin 5 (PfALV5), and a previously uncharacterized protein, referred to in this study as PhIL-1-interacting protein (PfPhIP). Co-localization of these proteins with PhIL-1 was observed using immunofluorescence assays and the formation of a second, PhIL-1-associated complex at the IMC that co-existed with the glideosome motor complex was identified. Finally, GlmS ribozyme knockdown of PfPhIP showed an 80% reduction in invasion and defective segmentation of daughter parasites during schizogony, demonstrated by Saini using electron microscopy. In conclusion, the research team identified a novel protein complex associated with the P. falciparum glideosome motor complex and showed that it is crucial in host cell invasion.
Maya Aleshnick (Oregon Health and Science University, USA) and her group have developed an alternative non-human primate relapsing malaria model that can be used for studying P. vivax, overcoming limitations of current P. vivax’s laboratory model. They used this model in P. cynamolgi, a Plasmodium spp. parasite that is genetically similar to P. vivax and develops a dormant hypnozoite stage in liver cells, a characteristic of P. vivax that is responsible for relapsing malaria infections. As a part of their experiments, Aleshnick and colleagues infected Anopheles mosquitoes with P. cyonomolgi parasites from rhesus macaques and have successfully observed exflagellation, oocyst formation and generation of salivary gland sporozoites. Further infection of rhesus macaques with these sporozoites was carried out and the course of infection in these non-human primates could be studied over time. Aleshnick also observed that drug treatment only cleared P, cynomolgi blood stages while the dormant hypnozoite stages persisted, resulting in the desired relapsing malaria model. This in vivo, non-human primate model allows for assessment of liver immunology and examination of hypnozoite and liver-stage parasites by minimally-invasive small liver biopsies. This group has also teamed up with other collaborators to perform single-cell sequencing of extracted cells to identify markers of hypnozoite formation. They are also working on developing P. cynomolgi strains that express P. vivax circumsporozoite protein (CSP) as a model for vaccine development.
Martha Cooper (Australian Institute of Tropical Health and Medicine, James Cook University, Australia) addressed the heterogeneity of human immune responses to P. falciparum infections and how these host differences might affect malaria parasites. In particular, Cooper’s research aims to identify baseline immune determinants of heterogenous infection outcomes. Using whole transcriptome and small RNA sequencing to quantify coding and noncoding RNAs following controlled human malaria infections (CHMI), Cooper identified a large number of differentially expressed RNA molecules, including coding-, long non-coding-, and micro- RNAs. Least Absolute Shrinkage and Selection Operator (LASSO) regression performed with re-sampled splitting, found a set of RNA molecules predictive of parasitemia and parasite multiplication rate after infection that accounted for about 50% of the variation in host immune responses. This work is a step toward understanding how particular immune responses control parasitemia and parasite multiplication rate after infection, which could ultimately enable rational vaccine design to elicit those specific responses.
Faith Osier (KEMRI – Wellcome Trust, Kenya and Heidelberg University, Germany) presented her research in identifying and robustly characterizing vaccine candidates, correlates of protection, and their mechanisms. Osier presented results from a controlled human malaria infection (CHMI) study in which volunteers were selected based on their previous malaria exposure as measured by seroreactivity against P. falciparum schizont extracts. Parasitemia was tracked daily using qPCR from day 7 onward after injection of P. falciparum (NF54) salivary gland sporozoites. It was found that individual infections separated into four groups: (1) those with exponential parasite growth, (2) individuals who were infected but eventually cleared parasites without treatment, (3) those who maintained consistent, low-level parasitemia, and (4), individuals who remained uninfected. Antibody levels against a panel of merozoite proteins were differential between these groups, with breadth of Fc activity against merozoite antigens positively associated with protection. These results suggest that focusing on a wide breadth of antigens that induce Fc-effector function could be beneficial for rational vaccine design.

Session 4 – Vector-Parasite interactions
This session was chaired by Damaris Matoke (Kenya Medical Research Institute (KEMRI), Kenya) who welcomed participants to the fourth session of the Women in Malaria conference on “Vector-parasite interactions”, where researchers explore interactions between the Plasmodium-infected mosquito and its microbiota for efficient control of malaria.
Nsa Dada (University of Abomey-Calavi, Benin) presented her research, which focuses on the mosquito microbiome and insecticide resistance. Dada began by highlighting that the previously declining rate of malaria morbidity and mortality worldwide is stagnating, and she attributed this to the global challenge of insecticide resistance and a stall in producing adequate vector control tools. Dada pointed to four main mechanisms of insecticide resistance: (1) mosquito cuticle modification, (2) increased metabolism, (3) target site alterations, and (4) behaviour change. Dada then introduced a lesser known mechanism: (5) microbial metabolism. Mosquitoes have microbes in them that regulate host physiology. Microbiota-mediated insecticide resistance is increasing in agricultural insect pests. Dada’s group questioned if the mosquito microbiota contributes to resistance or if it is affected by it. To unravel this mystery, they investigated if microbial composition differs between insecticide-resistant and insecticide-susceptible mosquitoes? Wild-caught Anopheles albimanus was exposed to fenitrothion and results showed that microbial compositions do differ between resistant and susceptible mosquitoes. They then aimed to investigate if these results would be similar in different locations of vectors and insecticides, and similar results were found; that insect pathogenic bacteria and bacterial antagonists were enriched in insecticide-susceptible mosquitoes. Altogether, Dada and colleagues showed that pyrethroid exposure alters mosquito microbiota; that mosquito microbiota differed by insecticide resistance phenotype, and that insecticide-metabolizing bacteria are enriched in the insecticide-resistant mosquito.
Bárbara Díaz Terenti (Institute of Parasitology and Biomedicine “López-Neyra” – IPBLN, Spain) began by mentioning that the success of the malaria transmission cycle relies on the interactions between the vector and the parasite. As such, Terenti and colleagues analyzed differential gene isoforms and alternative splicing mechanisms in the midguts and salivary glands of P. falciparum-infected and non-infected mosquitoes. Alternative splicing is a form of gene expression regulation that allows a single gene to code for more than one protein. It is known that P. falciparum infection of Anopheles mosquitoes causes changes in the metabolism of some nutrients in the mosquito, in the frequency of bites, in lower egg production, and in modulation of mosquito immunity. These changes are studied as changes in gene expression. However, the potential role of alternative splicing in mosquito phenotypes, such as vector competence, physiology and reproductive capacity, remained to be investigated until now. Through the work of Terenti and colleagues, hundreds of differentially-expressed gene isoforms were identified between P. falciparum-infected and non-infected Anopheles gambiae mosquitoes. Results also demonstrated that some isoforms are differentially expressed between infection of the midgut and salivary glands of mosquitoes. Most differentially-expressed isoforms were associated with vector-host interactions, including in the synthesis and metabolism of fatty acid and non-ribosomal peptides as well as immune responses. The most common alternative splicing mechanism observed in these experiments were exon skipping (ES) and the use of alternative transcriptional start sites (ATSSs). These results contribute to a better understanding of the importance of alternative splicing to the transcriptional response of mosquitoes when infected with the P. falciparum malaria parasite, and open the door to the design of new vector control strategies.
Sunita Swain (Tata Institute for Genetics and Society, India) defined An. stephensi as the most important urban malaria vector in India because of its high adaptation to its setting. Swain’s group aims to develop mosquitoes refractory to disease transmission. For this, they have assessed Anopheles population biology and genetics by bringing larvae and adult mosquitoes from different parts of India and maintaining them in insectary conditions with the purpose of evaluating the vectorial capacity of different mosquito eco-types. This study also compared the susceptibility of An. stephensi L. populations to Plasmodium infection. Of 4 mosquito colonies, termed Types 1-4 (T1-4), one population (T4) has already been cultivated for more than 120 generations. The other 3 populations have surpassed 50 generations since their collection. Wild mosquitoes from T1 and T4 locations were resampled and their DNA was extracted for molecular studies in 2019, with no difference in fitness observed. Microbiota was observed to be influenced by the habitat and food intake of the mosquitoes. The ecological adaptation of An. stephensi complexifies its spatial distribution. Despite the high differential susceptibility of these vectors for Plasmodium falciparum, the susceptibility for Plasmodium berghei remains low among colonized populations. At present, Swain and colleagues have observed that the conditions of larval rearing, age of mosquito during feeding with Plasmodium spp.-infected blood, and genetic diversity all contribute to alterations in vectorial capacity of the mosquito.
Paola Carrillo-Bustamante (Max Planck Institute for Infection Biology, Germany) discussed the creation of an individual-based mathematical model for Plasmodium transmission that takes into account human, mosquito, and parasite factors. Simulated malaria infection shows infected mosquitoes demonstrate slow growth, reaching 20% in 300 days. The model predicts transmission events are rare and caused by long-lived mosquitoes. Recently emerged mosquitoes have a low metabolism and blood meal increases the mosquito metabolism by increasing reserves, egg laying capacity and parasite development. These parameters are positively correlated to the number of blood meals. Long sporogony is beneficial under multiple feedings. The model suggests that transmission events are extremely rare and caused by long-lived mosquitoes that feed multiple times. Plasmodium successfully competes for resources accumulated during multiple blood meals. In their next steps, they will check if malaria parasites exploit mosquitoes that feed several times to optimise transmission and if in those cases the long EIP is advantageous.
Flaminia Catteruccia (Harvard T.H. Chan School of Public Health, USA) and her group focuses on studying the interaction between mosquito reproduction and parasite development. Catteruccia’s talk started with some information on the vector-parasite cycle, with emphasis on infected mosquito blood-feeding and on the location of parasite development (midgut) and mosquito egg development (ovaries). In this study, Catteruccia and colleagues aimed to investigate if parasite development affected egg development in Anopheles gambiae. Results showed the number of mosquito eggs was not affected by the presence of the parasite. A positive correlation between egg development and the number of oocysts was also drawn when comparing both lab and wild-caught mosquitoes. The hydrosteroid synthesised 20E by female mosquitoes after blood feeding was found to be interfering with egg development. In fact, interfering with steroid hormones reduces the number of eggs and the number of parasites, resulting in the death of the parasite when egg development is interrupted. Females in which the 20E cycle is interrupted have bigger oocysts due to faster parasite growth and also a higher number of sporozoites in salivary glands. In addition, it was found that impairing egg development increases the speed of Plasmodium falciparum oocyst development in the mosquito. The parasite is capable of utilising lipids transported by lipophorin proteins and can export nutrients from mosquitoes. Subsequent blood meals increase the speed of parasite growth and the extrinsic incubation period (EIP). Catteruccia ended the talk mentioning the antimalarial atovaquone. They observed that mosquitoes exposed to atovaquone had no parasites, showing that antimalarials can kill parasites within mosquitoes. This strategy was tested against insecticide resistant An gambiae, and it was found to be 100% efficient against insecticide resistant mosquitoes and parasites in that area.
This report is brought to you by the MESA Correspondents Sushma Ambekar (Iowa State University, USA), Christine Markwalter (Duke Global Health Institute, USA), Rosheen Mthawanji (Malawi Liverpool Wellcome Trust, Malawi) and Nkahe Diane Leslie (University of Yaoundé I, Cameroon). Senior editorial support has been facilitated by Joanne Power (Pennsylvania State University, USA).
Published: 22/03/2021
This report is brought to you by the MESA Correspondents Sushma Ambekar, Christine Markwalter, Rosheen Mthawanji, and Diane Leslie Nkahe. Senior editorial support has been facilitated by Joanne Power, Elena Gómez Díaz and Sarah Reece.
THEMES: Basic Science | Epidemiology | Health Systems