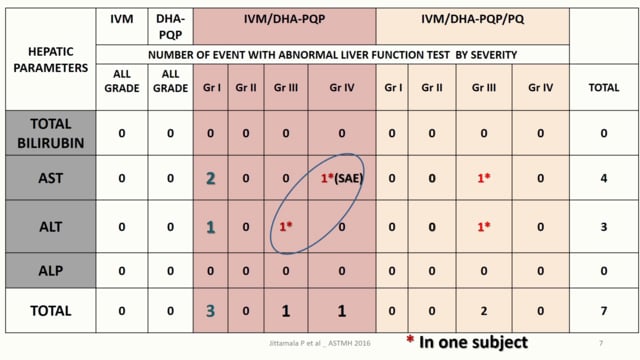
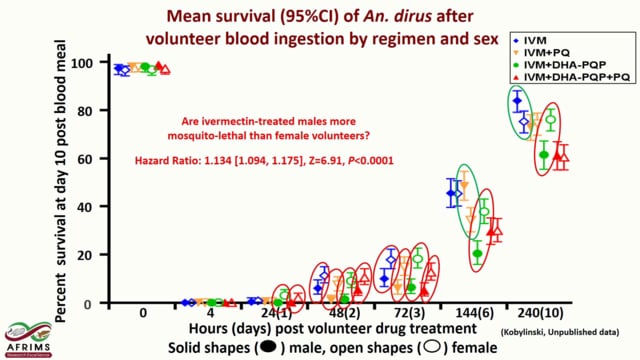
Last Updated: 02/12/2024
Safety, tolerability and efficacy of PfSPZ Vaccine against heterologous controlled human malaria infection in healthy adults
Objectives
The aim of this study is to determine if individuals living in a non-malaria endemic area such as the United States (US) are protected against heterologous CHMI conducted at these time points.
USSPZV7 is a randomized, phase 1, double-blind, placebo-controlled trial of Sanaria® PfSPZ Vaccine administered on Days 1, 8 and 29 by direct venous inoculation (DVI) to assess safety, tolerability, immunogenicity, and vaccine efficacy (VE) against heterologous controlled human malaria infection (CHMI) with the 7G8 clone of Plasmodium falciparum (Pf) conducted at 3 or 12 weeks after the third immunization.
Study type: Interventional
Enrollment: 60 participants
Primary purpose: Prevention
Allocation: Randomized
Interventional Model: Parallel assignment
Masking : Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
NCT number: NCT05604521
Phase: Phase I
Article: A PfSPZ vaccine immunization regimen equally protective against homologous and heterologous controlled human malaria infection Article: Multidose Priming and Delayed Boosting Improve Plasmodium falciparum Sporozoite Vaccine Efficacy Against Heterologous P. falciparum Controlled Human Malaria Infection