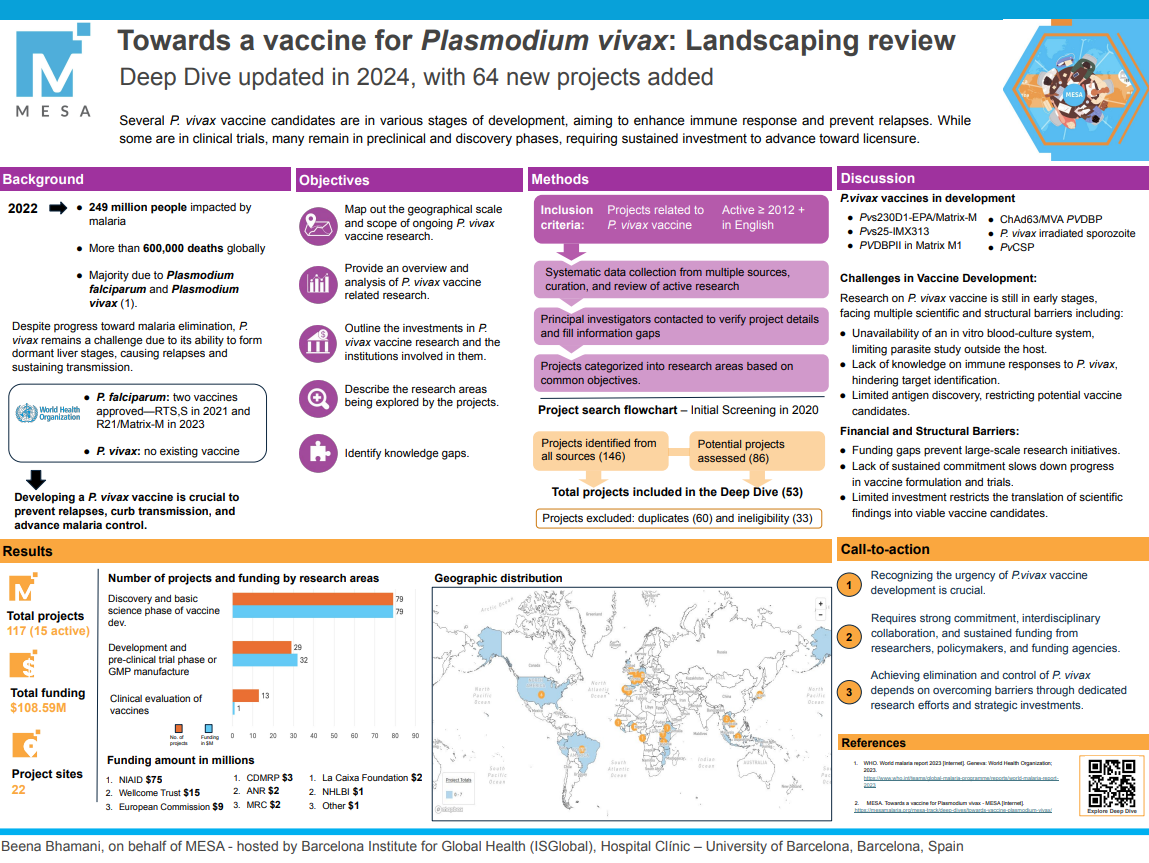
Last Updated: 02/12/2024
Safety, immunogenicity, and transmission-blocking activity of P. vivax vaccine Pvs230D1-EPA/Matrix-M in health malaria-naïve adults
Objectives
The aim of this study is to test the effects of a new malaria vaccine. (Volunteers will not be exposed to malaria.)
Malaria is a disease carried by mosquitoes in tropical countries around the world. It can cause symptoms like fever, body aches, and weakness. More than half a million people worldwide died of malaria in 2021, mostly children. Researchers want to find ways to prevent the spread of this disease.
Eligibility:
Healthy adults aged 18 to 50 years.
Design:
Volunteers will be screened. They will have a physical exam with blood and urine tests. They will take a short quiz to make sure they understand the study.
Volunteers will have 3 visits to receive the vaccine. These visits will be about 1 month apart. The vaccine will be injected into the muscle of the upper arm.
Volunteers will have 12 additional clinic visits. These will start after the first vaccine visit and continue for 8 months. The visits may include a physical exam and blood tests. There will also be 7 follow-up phone calls. These will occur the day after each vaccine visit and then continue for another 12 months. Participants will be asked how they are doing and whether they have had any changes in their health.
Study type: Interventional
Enrollment: 200 participants
Primary purpose: Prevention
Allocation:Non-Randomized
Interventional Model: Sequential assignment
Masking :Open label
NCT number: NCT05913973
Phase: Phase I
Aug 2023 — May 2025