Last Updated: 19/06/2023
Rapid, point of care diagnostic for malaria, highly sensitive for P. vivax, with species differentiation
Objectives
To develop a one-minute, $1, point-of-care diagnostic that has a limit of detection for P. vivax >5x lower than RDTs.
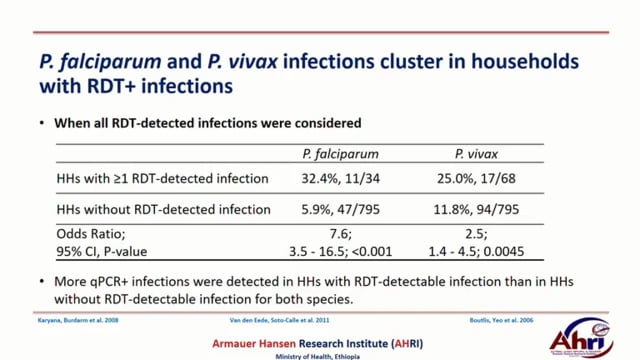
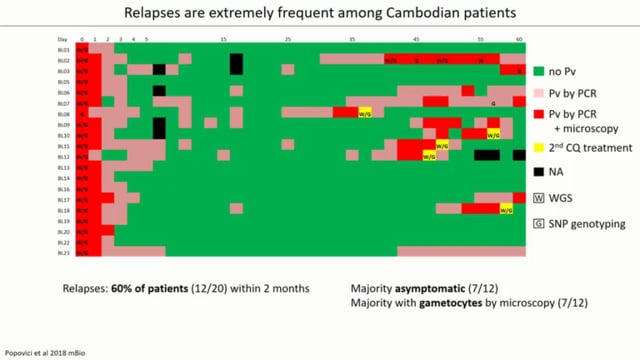
While significant progress has been made in reducing malaria prevalence and deaths, malaria elimination remains an extremely difficult goal. P. falciparum and P. vivax species of malaria parasites account for ~95% of all human malaria infections. P. falciparum is more virulent and has a higher incidence of adverse clinical outcomes, but P. vivax is more pervasive and widespread, often becoming the predominant species in regions moving toward elimination. These parasites follow a different course of disease and the treatment regimen differs, requiring an additional drug for P. vivax to eliminate a liver stage which causes disease relapse potentially years after infection. Current diagnostics have suboptimal accuracy, especially for P. vivax, as well as delayed turnaround times. This diagnostic platform will also have the ability to differentiate between P. falciparum and P. vivax to allow for appropriate treatment. The proposed project advances the current rapid malaria detection device, which detects the presence of any malaria parasites in patients with >95% accuracy compared to microscopy. The new capability will be commercialized into a robust, portable, point-of-care product. It has the potential to greatly reduce malaria transmission and could substantially contribute towards reduction in human suffering as well as help to achieve malaria elimination.
Aug 2020 — Jul 2022
$1.67M