Last Updated: 09/12/2024
Multivalent mRNA-based malaria vaccines
Objectives
The proposed study will test the whether a mRNA-based vaccine can be optimized to concurrently drive durable, antibody-mediated and cell-mediated protective immune responses to pre-erythrocytic and blood-stage malaria vaccine candidates.
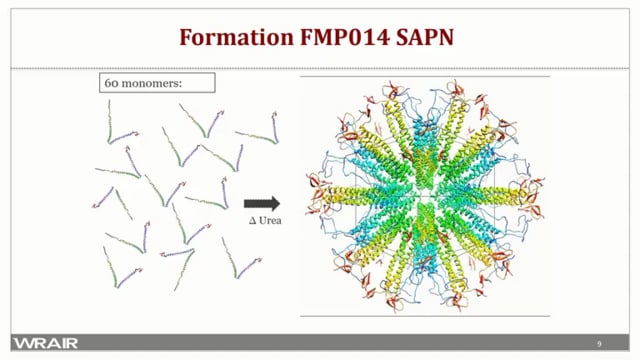
The heavy burden of Plasmodium falciparum and Plasmodium vivax malaria drove international efforts to coordinate and integrate tools and programs for mosquito vector control, rapid diagnosis, and drug treatment. Success has been significant but may be approaching a limiting boundary. The effort to develop a vaccine to further enhance malaria control has faced many challenges. The advancement of the pre-erythrocytic-stage RTS,S vaccine is a major accomplishment, providing the foundation for an effort that must continue to improve efficacy and durability of vaccine-induced protection to further reduce the disease burden. In this regard, the development of a multivalent vaccine that concurrently targets pre-erythrocytic-stage, blood-stage and sexual stage parasites is attractive but adds complexity. The immunogenicity of each component must be maintained while effectively driving both antibody and cell-mediated immune responses. A recombinant antigen plus adjuvant has been utilized approach to systematically formulate an immunogenic, multi-stage, multivalent malaria vaccine made feasible through the use of an optimized malaria-specific carrier protein, P. falciparum merozoite surface protein 8. While successful, there are still challenges and limits to this approach considering the breadth and nature of immune responses that are needed. Several features of the emerging mRNA vaccine technology are extremely attractive for malaria vaccine development and may overcome challenges inherent to producing multiantigen, multistage formulations. At the same time, the technology must be evaluated and optimized to address issues unique to Plasmodium and malaria. This project will optimize mRNA vaccine-induced, antibody-mediated, and cell-mediated protection to pre-erythrocytic stage parasites using a novel P. falciparum circumsporozoite protein (PfCSP) vaccine candidate. The project will design, produce, and evaluate an mRNA-based vaccine targeting P. falciparum reticulocyte-binding protein homologue 5 (PfRh5) to drive high titer antibodies that block merozoite invasion of erythrocytes. Data will be applied on a highly protective, Plasmodium yoelii mRNA blood-stage vaccine to the design and testing of a vaccine targeting the 19 kDa C- terminal domain of P. falciparum merozoite surface protein 1 (PfMSP119). A multivalent mRNA- based vaccine will be evaluated that concurrently targets sporozoites and liver-stage parasites (PfCSP) as well as blood-stage merozoites (PfRh5 and PfMSP119). Success in the effort will determine the potential and the limitations of mRNA- based technology for delivery of a multicomponent malaria vaccine and provide a foundation to integrate additional targets and/or modifications to advance development of a true multi-stage malaria vaccine.
Aug 2024 — Jul 2029
$738,445