Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Last Updated: 02/12/2024
Malaria vaccine evaluation in a novel infant non-human primate (NHP) challenge model
Objectives
To develop a novel infant rhesus malaria challenge model and use this model to determine how age impacts immune responses to malaria vaccination and protection at challenge.
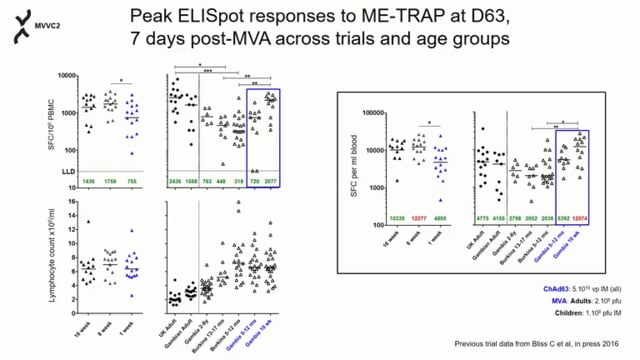
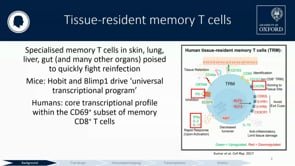
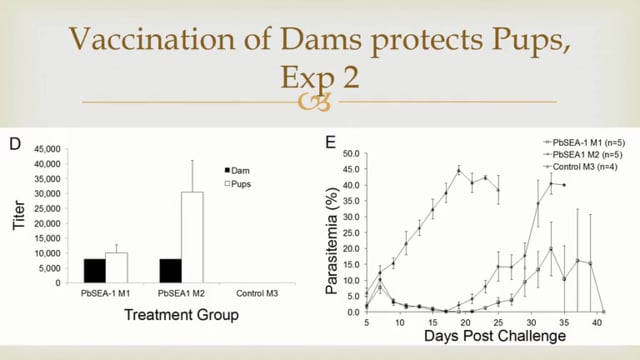
Malaria, caused by Plasmodium parasites, is responsible for 225 million infections and 400,000 deaths per year. Malaria burden is unevenly distributed by age, with infants and children suffering most. There is currently no licensed malaria vaccine that can provide high level efficacy and reduce transmission in these populations. The most advanced vaccine candidates target the malaria sporozoite and liver stages, which precede the symptomatic blood stage of infection. These include the subunit vaccines RTS,S, R21, and ME-TRAP, and the live-attenuated sporozoite vaccine PfSPZ. These vaccines confer protection by inducing anti-sporozoite antibodies or cytotoxic T cells that kill infected cells in the liver. While these candidates have shown promising vaccine efficacy in adults, age de-escalation trials have revealed vaccine immunogenicity and efficacy vary with age, with a trend for poorer vaccine efficacy in younger infants. This has been attributed to the inherently tolerogenic nature of the infant immune system, which gradually changes as the individual ages and becomes more immunologically mature. Non-human primates (NHPs) are excellent models of the human immune system and pre-clinical studies in adult rhesus macaques have directly informed malaria vaccine clinical trials. For vaccines that induce liver-specific T cell responses, NHPs have been particularly valuable for identifying tissue correlates of protection since the liver is typically inaccessible in vaccine clinical trials. Yet, despite infants and children being the target population for malaria vaccines, there is currently no infant NHP challenge model for malaria vaccine evaluation. This proposal will address this critical gap by establishing a novel infant rhesus malaria challenge model and using it to define the effect of age on immune responses to malaria vaccination and protection at challenge. This model will use the NHP-adapted Plasmodium knowlesi (Pk) malaria parasite and the well-characterized PkSPZ live-attenuated sporozoite vaccine, which is equivalent to the PfSPZ vaccine recently tested in human infants. This vaccine strategy is ideal as it is known to induce antibodies and T cell responses in both the periphery and liver and confer substantial protection in adult rhesus macaques. The study design will age de-escalate and enroll one cohort young adult animals and another of 5-12 month old infants. The hypothesis is that age-dependent differences in peripheral and liver-specific immune responses to vaccination will result in decreased vaccine efficacy in the infants. As it is infeasible to study vaccine-induced T cell responses in the liver of human infants, this study will be the first to assess how the inherent characteristics of the developing infant immune system impact liver T cell responses in a translationally relevant model. These studies aim to launch this novel infant NHP research model and accelerate the development of a highly effective malaria vaccine for human infants.
Nov 2022 — Oct 2024
$353,000