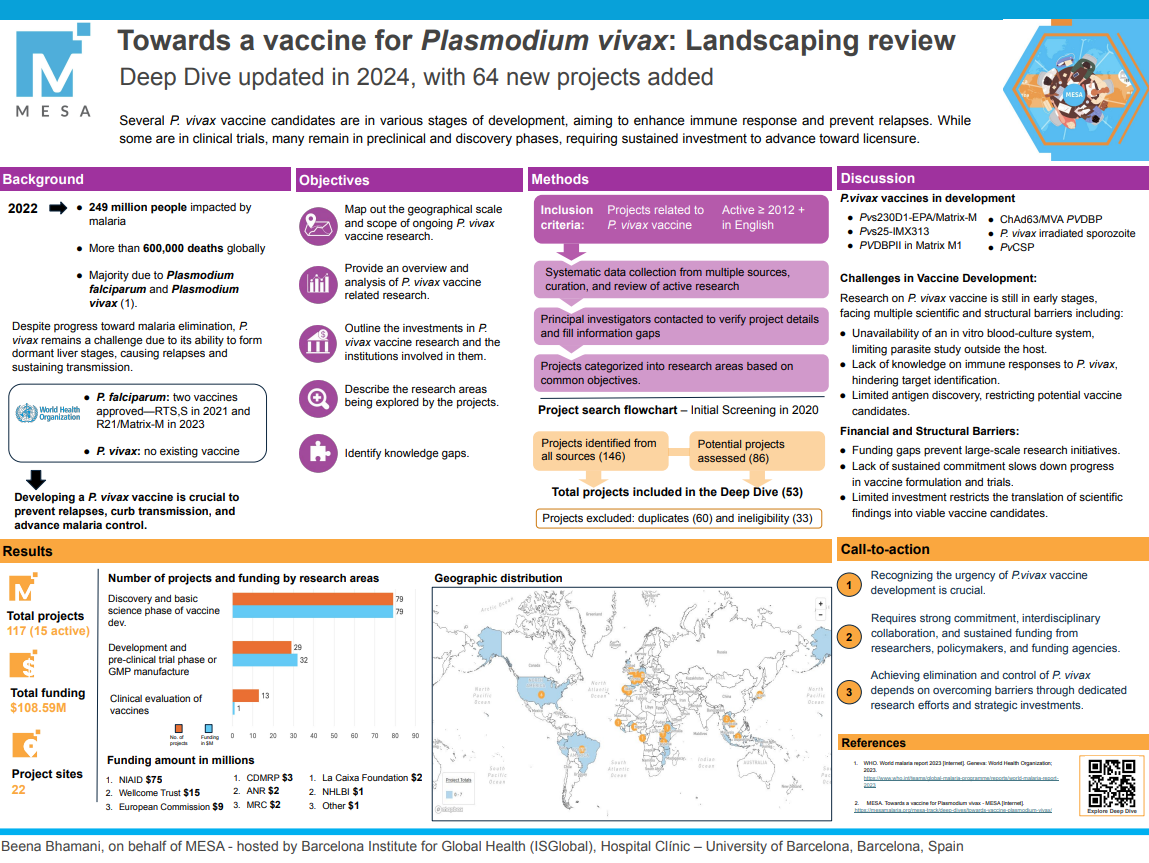
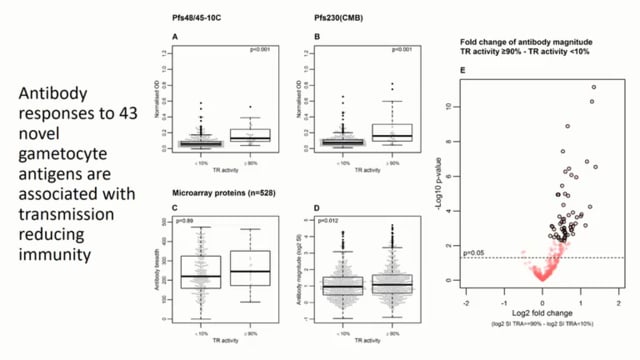
Last Updated: 02/12/2024
A vaccine targeting eradication of malaria (UltiMalVax)
Objectives
This proposal sets to develop such a vaccine assessing both established virus-like particle (VLP) vaccines in potent saponin adjuvants and also exciting new thermostable mRNA vaccines expressing the parasite antigens now showing high efficacy.
Malaria killed about 640 thousand people in 2020, largely young children in Africa. Rapid recent progress has led to two anti-sporozoite vaccine developers planning WHO prequalification applications in 2022. These include the new high efficacy R21/Matrix-M vaccine, to be supplied at the required large scale, and led by partners in this consortium. In parallel, recent progress with transmission-blocking malaria vaccines has led to substantial efficacy in a first direct skin feeding field trial. This opens up the prospect of a two-stage vaccine targeting both sporozoites and sexual-stage parasites that should have a major impact on malaria transmission, thereby enabling regional elimination and ultimate eradication. Importantly, new VLP design technologies, e.g. SpyCatcher bonding will be adopted that allow bivalent antigen display, to enable a single vaccine to protect against both the Plasmodium falciparum parasite, which causes most deaths, and the more widespread Plasmodium vivax parasite. A lead vaccine candidate will be down-selected based on well-studied pre-clinical efficacy models and induction of functional transmission-blocking antibodies, prior to GMP manufacture and a clinical trial in year 4. The consortium brings together academics, non-profits and a wide range of companies with both leading technologies and access to small and very large scale GMP manufacturing capacity. This programme builds on the recent success of several partners in the R21/Matrix-M programme and aims to accelerate the malaria eradication agenda by providing the first vaccine to tackle both major malaria parasite species, and confer both individual and community protection on the way to eradication.
May 2023 — Apr 2027
$2.83M