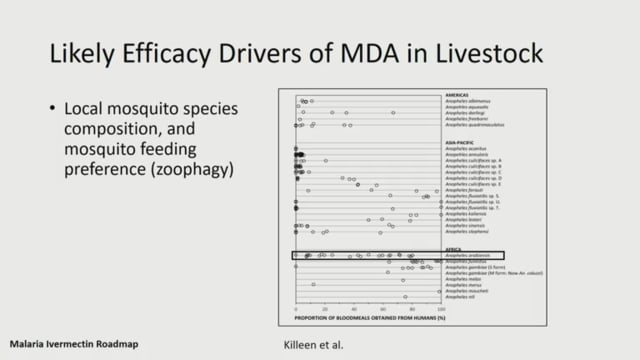
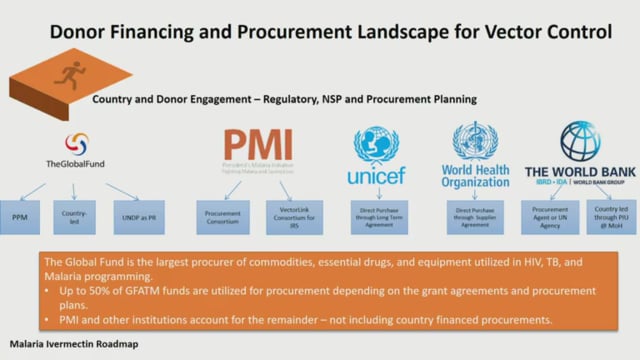
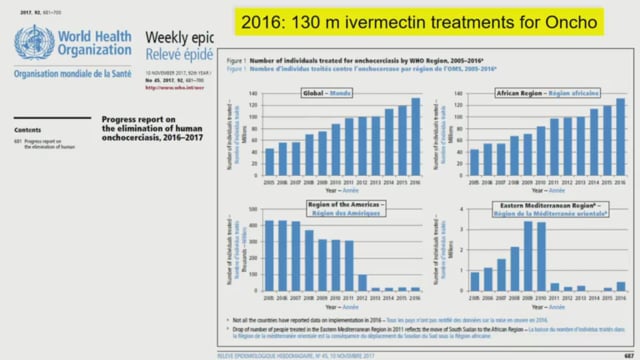
ASTMH 2018, Cassidy L. Rist: “Ivermectin Roadmap: enhancing impact from One Health strategies”
Collaborator(s): Virginia Tech, United States
Published: 29/10/2018
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 67th ASTMH annual meeting in New Orleans, October 2018
Title: “Ivermectin Roadmap: enhancing impact from One Health strategies“
Speaker: Cassidy L. Rist, Virginia-Maryland College of Veterinary Medicine at Virginia Tech, Blacksburg, VA, United States
Session information:
Symposium 30: A Roadmap for Ivermectin as a Complementary Vector Control Tool for Malaria
October 29, 2018, 1:45 PM – 3:30 PM, Sheraton – Grand Ballroom C (5th Floor)
Abstract:
Ivermectin is a licensed drug with an excellent safety profile that has been broadly used against human onchocerciasis, lymphatic filariasis and other Neglected Tropical Diseases (NTDs) through intermittent, single dose, Mass Drug Administration (MDA) Campaigns, as well as treatment of helminths in cattle. The recognition that mosquitos that feed on humans and other mammals treated with ivermectin suffer both direct (death) and indirect (changes in behaviour) effects has led to increased interest in the potential use of ivermectin as an adjunct vector control tool for malaria, particularly in the context of residual transmission. A Preferred Product Characteristics document (PPC) was published by WHO, and Ivermectin is now included in the range of priority TPPs for Medicines for Malaria Venture as TCP6. However, the malaria community still lacks clarity on critical issues related to the development pathway, particularly the dose and drug regimen, as well as the specific studies, guiding regulatory processes and policy pathway that would lead to licensure and effective use of ivermectin as a complementary vector control tool to reduce malaria transmission. There are a small number of ongoing trials with varying designs, regimens, endpoints, and sources of funding. Should it meet all milestones, the pathway to financing ivermectin needs to be established for the malaria indication, since it is currently donated for NTDs MDAs. In addition, clarification of clinical and regulatory pathway could facilitate development of novel candidates that could offer superior performance (longer half-life, for example) in the longer term. ISGlobal is currently leading a process to bring the community together to develop the Ivermectin Roadmap as a novel tool for malaria. The Roadmap reflects the work of 35 experts in their fields, covering clinical trials, entomology, drug development, vector tool development, malaria and veterinary medicine, ethics, industry, social scientists, program implementers, past NMCP directors, NTD specialists, and modellers. This symposium will present the results of this process including: (a) the proposed roadmap (b) key issues to be resolved (c) proposed go/no-go criteria.