ASTMH 2018, Tamsin E. Lee: “Identifying key factors of the transmission dynamics of drug-resistant malaria”
Collaborator(s): Swiss Tropical and Public Health Institute (Swiss TPH), Switzerland
Countries: Switzerland
Published: 30/10/2018
In collaboration with the poster presenters, MESA brings you this sneak-peak of poster 1085 from the 67th ASTMH annual meeting in New Orleans, October-November 2018
Title: “Identifying key factors of the transmission dynamics of drug-resistant malaria”
Speaker: Tamsin E. Lee, Swiss Tropical and Public Health Institute, Switzerland
Abstract:
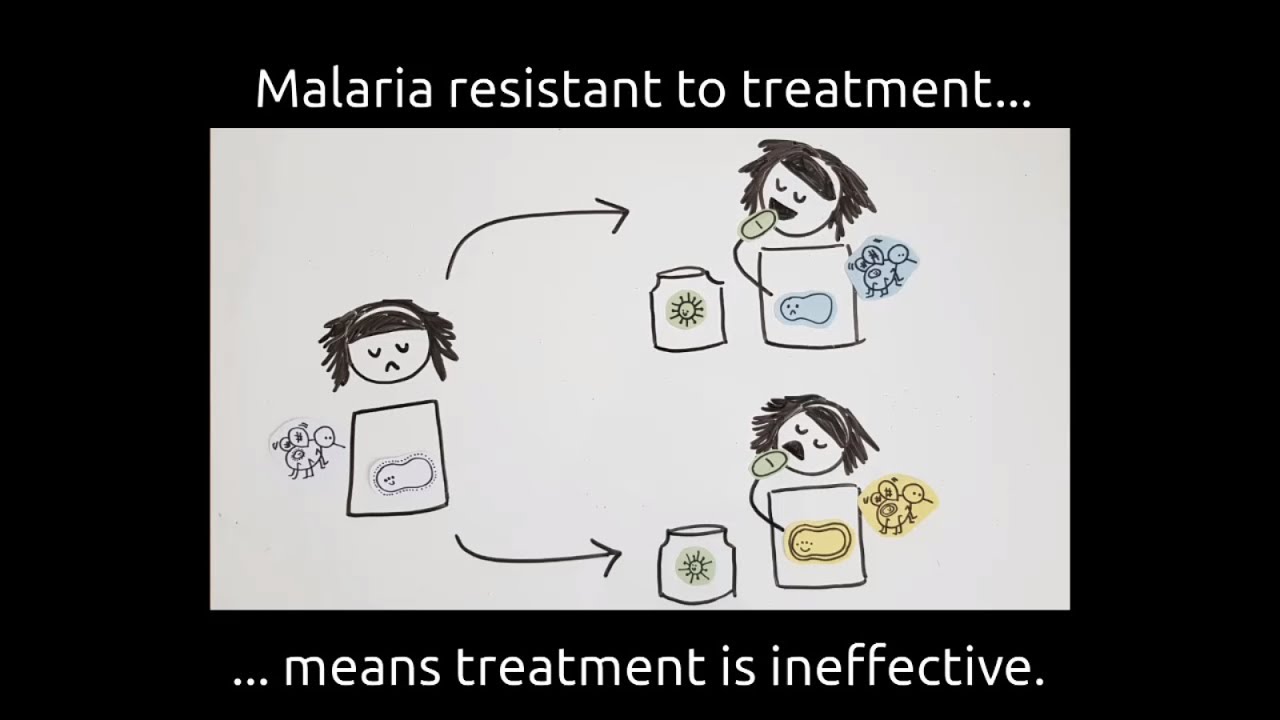
Development of resistance to malaria treatments remains a great threat to malaria control and continued transmission reduction. Understanding the key factors that increase the spread of drug resistance or emergence can guide intervention strategies. In particular, to inform risk versus benefits assessments in regards to mass treatment strategies and their potential impact on resistance in different settings. Mathematical modelling provides a framework to understand factors which contribute to the spread of malaria, and importantly can simulate specific drugs and different treatment strategies. The simplicity and relevance of the Ross-MacDonald has ensured that it continues to be a strong basis for a broader theory of mosquito-borne disease transmission and control. We present a model that builds upon these well-established foundations to understand drivers of resistance. Our model includes three infection classes: sensitive, partial resistance and fully-resistant. These classes are flexible and could represent a single infection with an intermediary step to full-resistance, or each infected host carries two infections where one may be sensitive and the other resistant. Via sensitivity analysis of the model we find that the most
effective way to control the spread of resistant infections is by interrupting the rate that more resistant infections replace less resistant infections, and as such this mechanism should be the focus of further research. Importantly, our results imply that reducing treatment has a comparatively small impact, since the most effective way to prevent resistance spreading throughout a population is by controlling the within-host mechanisms of resistance via pharamacokinetics. Our model serves as an introductory model that can act as the foundation for further studies of resistance drivers in a population, and compliment and inform within-host models of resistance.
THEMES: Drug Resistance | Measurement of Transmission | Modeling