ASTMH 2017, Anais Bompard: “High Plasmodium falciparum oocyst loads in naturally infected mosquitoes in Africa”
Collaborator(s): Research Institute for Development (IRD), France, France
Countries: Burkina Faso, Cameroon
Published: 09/11/2017
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 66th ASTMH annual meeting in Baltimore, November 2017
Title: “High Plasmodium falciparum oocyst loads in naturally infected mosquitoes in Africa”
Speaker: Anais Bompard, Institute of Research for Development (IRD), France
Session information:
Symposium 0175: “Malaria: Mosquito Transmission and Interruption”
Thursday, 9 November, 8:00 – 9:45 AM, Convention Center – Ballroom II (Level 400)
Abstract:
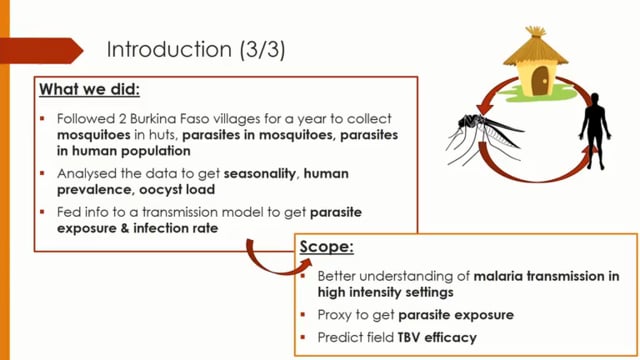
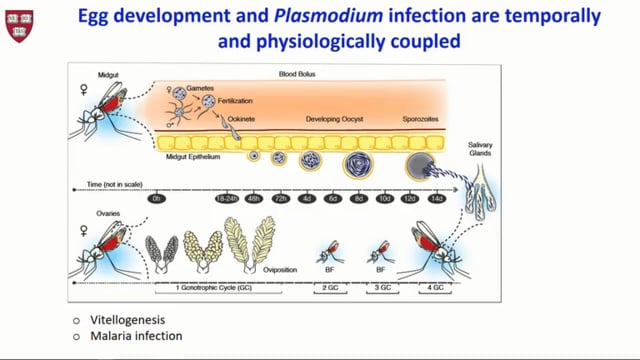
The population dynamics of human-to-mosquito malaria transmission in the field has important implications for the genetics, epidemiology and control of malaria. The number of Plasmodium falciparum oocysts in naturally infected mosquitoes in the wild is poorly understood, though past work indicates that most mosquitoes have only one or two oocysts. The per bite mosquito force of infection (the mean number of oocysts gained from an infectious bite) is also unclear despite the force of infection influencing factors such as the efficacy of novel transmission blocking interventions currently under development. Here a yearlong analysis of malaria transmission in three sites in Burkina Faso and Cameroon is reported. Naturally fed mosquitoes were caught inside houses and dissected to assess the prevalence and intensity of oocysts and sporozoites 3 and 7 days after collection. Cross-sectional surveys of the resident human population were carried out to determine the prevalence and intensity of sexual and asexual parasites. Results show that oocysts intensity in naturally infected mosquitoes is substantially higher than previous estimates. In the rainy season infected mosquitoes had on average 9-18 oocysts per mosquitoes 3 days after collection, with one mosquito harboring 786 oocysts. Multivariate analysis indicated that village, season and bednet use in the local population to be associated with the prevalence and intensity of oocysts and the sporozoite rate. A dynamical mathematical model of transmission was used to estimate the per-bite transmission probability, the proportion of superinfections and average parasite exposure per bite for each location. The implications of high parasite exposure on biology of transmission and the development and use of transmission blocking interventions in the field are discussed.
THEMES: Measurement of Transmission