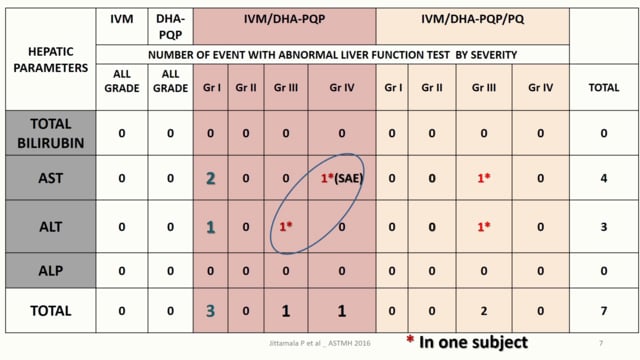
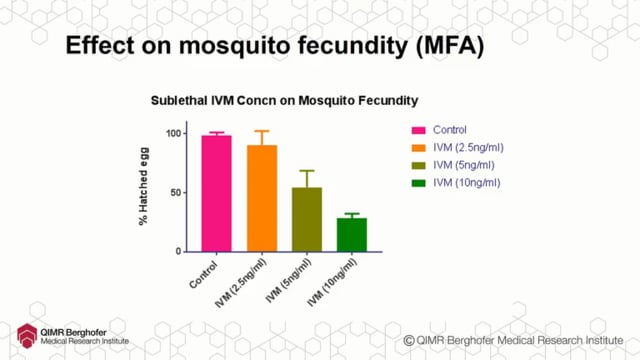
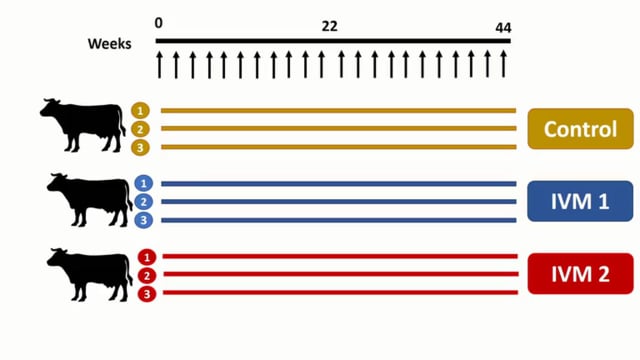
ASTMH 2016, Podjanee Jittamala:”Safety and mosquito-lethal efficacy of ivermectin, dihydroartemisinin-piperaquine and primaquine: Ivermectin for malaria in Southeast Asia (IMSEA Study, Thailand)”
Collaborator(s): Mahidol University, Thailand
Published: 15/11/2016
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 65th ASTMH annual meeting in Atlanta, November 2016.
Title: “Safety and mosquito-lethal efficacy of ivermectin, dihydroartemisinin-piperaquine and primaquine: Ivermectin for malaria in Southeast Asia (IMSEA Study, Thailand)”
Speaker: Podjanee Jittamala, Faculty of Tropical Medicine, Mahidol University
Session information: Symposium 69: “Ivermectin to Reduce Malaria Parasite Transmission: Clinical Trials, Models, and Regulatory Pathways to Accelerate Implementation”
Tuesday, 15 November, 10:15am – 12:00 pm, Marriott – Marquis B
Abstract:
Novel vector control tools are urgently needed to aid malaria elimination efforts worldwide. Although insecticide treated bednets and indoor residual spraying have dramatically reduced malaria transmission burden, it has become clear that these measures alone cannot thwart all transmission due to vectors that feed outside the home or outside the period of time that persons are protected by bed nets. Ivermectin, an anthelminthic drug widely taken in malaria endemic regions, has been shown to reduce the survivorship of Anopheles vectors taking bloodmeals containing the drug. Ivermectin mass drug administrations (MDAs) in West Africa reduced wild Anopheles gambiae survivorship and the proportion of vectors with Plasmodium falciparum. Ivermectin MDA is a promising new tool as it targets vectors at the point of blood feeding, regardless of Anopheles temporal or spatial blood feeding behaviors. Thus, ivermectin MDA has the potential to work in a diverse range of ecological settings and should be evaluated in multiple malaria transmission settings throughout the world. Here we present results from three clinical trials being conducted in Burkina Faso, Kenya, and Thailand to assess the potential effectiveness of ivermectin for malaria parasite transmission suppression. Each trial assesses different drug regimens and critical Anopheles vectors found worldwide. The field MDA trial in Burkina Faso is the first to assess the impact of repeated ivermectin (200 µg/kg) MDAs on both mosquito and human Plasmodium transmission outcomes. The clinical trial in Kenya is the first to assess the safety, tolerability, pharmacokinetic interaction and mosquito-lethal efficacy of three daily doses of ivermectin (300 or 600 µg/kg/day) in conjunction with dihydroartemisinin-piperaquine against An. gambiae. The clinical trial in Thailand is the first to assess the safety, tolerability, pharmacokinetic interaction and mosquito-lethal efficacy of single-dose ivermectin (400 µg/kg) in conjunction with dihydroartemisinin-piperaquine and primaquine against An.dirus and An. minimus. As the use of antimalarial MDA has been increasing, it is important to evaluate the safety of ivermectin with these antimalarial drugs as their simultaneous deployment could have dramatic impact on malaria incidence. Some of these trial results have been incorporated into robust malaria transmission models to better understand the most appropriate use of ivermectin MDA in a malaria elimination context. Finally, the symposium will discuss some of the regulatory and policy issues surrounding the use of ivermectin MDA for malaria parasite transmission control.
THEMES: Drug-based Strategies | Vector Control