ASTMH 2016, Stephen A. Kaba: “Humoral Immune Responses to an Adjuvanted Self-Assembling Protein Nanoparticle (SAPN) Malaria Vaccine Displaying the NANP repeat and αTSR regions of the Plasmodium Falciparum Circumsporozoite Protein”
Collaborator(s): Walter Reed Army Institute of Research (WRAIR), United States
Published: 14/11/2016
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 65th ASTMH annual meeting in Atlanta, November 2016.
Title: “Humoral Immune Responses to an Adjuvanted Self-Assembling Protein Nanoparticle (SAPN) Malaria Vaccine Displaying the NANP repeat and αTSR regions of the Plasmodium Falciparum Circumsporozoite Protein”
Speaker: Stephen A. Kaba, Walter Reed Army Institute of Research, USA
Session information: Scientific Session 34: Malaria: Vaccines – Diverse Approaches
Monday, 14 November, 1:45 – 3:30pm, Marriott – Marquis C
Abstract:
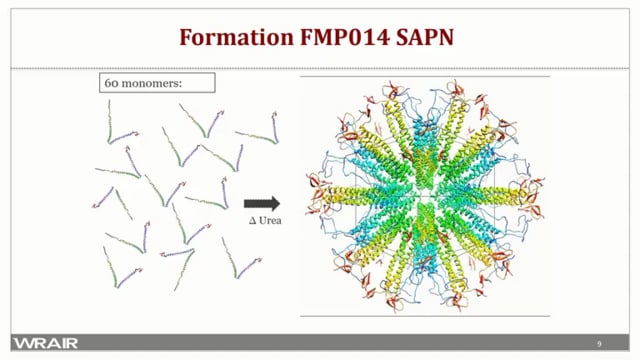
The Circumsporozoite Protein (CSP) is the predominant protein on the surface of the sporozoite and the most thoroughly studied pre-erythrocytic vaccine candidate. It is composed of an N-terminal domain, a conserved pentapeptide protease cleavage site termed region I, a tetra peptide NANP repeat region, a short conserved sequence termed region III, and a C-terminal region with sequence homology to the thrombospondin type-1 repeat superfamily (TSR). We have previously reported that a self-assembling protein nanoparticle, PfCSP-KMY-SAPN, presenting NANP repeats and three human HLA epitopes of the CSP induced very strong protective immune responses in mice. However, when tested in NHP, immune responses were low even when an adjuvant was used. To improve the vaccine induced immune response we have produced FMP014, a SAPN displaying NANP repeats and the entire αTSR domain of CSP. The αTSR in the SAPN uses two Cys-Cys disulfide bonds to stabilize the hydrophobic pocket and core link found in the native protein. Here we report the analysis of the humoral immune responses of C57Bl/6 mice to FMP014 combined with three different Army Liposomal Formulations containing monophosphoryl lipid A with or without QS-21, and Alhydrogel® (ALFA, ALFQ and ALFQA). Intramuscular injection of C57Bl/6 mice with each FMP014/ALF formulation induced high titer anti-NANP repeat, anti-αTSR domain, and cytophilic IgG2b antibodies with high avidity. However, FMP014 adjuvanted with ALF containing QS-21 (ALFQ) induced significantly higher anti-CSP specific antibodies to the NANP repeats and the αTSR domain compared to those without QS-21. Antibodies to both the NANP repeat region and the αTSR domain conferred protection to mice against challenge from an otherwise lethal dose of a transgenic P. berghei parasite expressing the full-length P. falciparum CSP.
THEMES: Enabling Technologies & Assays | Vaccines