ASTMH 2016, Sophie Roetynck: “Immunogenicity of ChAd63/MVA ME-TRAP malaria vectored vaccine is not affected by co-administration with routine EPI vaccines in a randomized controlled trial in Gambian infants and neonates”
Collaborator(s): Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene and Tropical Medicine, The Gambia
Countries: The Gambia
Published: 14/11/2016
In collaboration with ASTMH, Image Audiovisuals, and session presenters, MESA brings you this webcast from the 65th ASTMH annual meeting in Atlanta, November 2016.
Title: “Immunogenicity of ChAd63/MVA ME-TRAP malaria vectored vaccine is not affected by co-administration with routine EPI vaccines in a randomized controlled trial in Gambian infants and neonates”
Speaker: Sophie Roetynck, Medical Research Council Unit, The Gambia
Session information: Scientific Session 34: Malaria: Vaccines – Diverse Approaches
Monday, 14 November, 1:45 – 3:30pm, Marriott – Marquis C
Abstract:
Recent global estimates show that P. falciparum malaria remains a major public health concern. An effective vaccine could complement existing control measures. Heterologous prime-boost vaccinations using chimpanzee adenovirus 63 (ChAd63) and modified vaccinia Ankara (MVA) encoding ME-TRAP have consistently shown acceptable safety, excellent immunogenicity and substantial efficacy in African adult and paediatric populations. If licensed, this vaccine will be given to infants, who receive routine childhood immunizations. Here, we evaluate the immunogenicity and possible interference of ChAd63/MVA ME-TRAP when co-administered with routine Expanded Programme Immunization (EPI) vaccines in young infants.
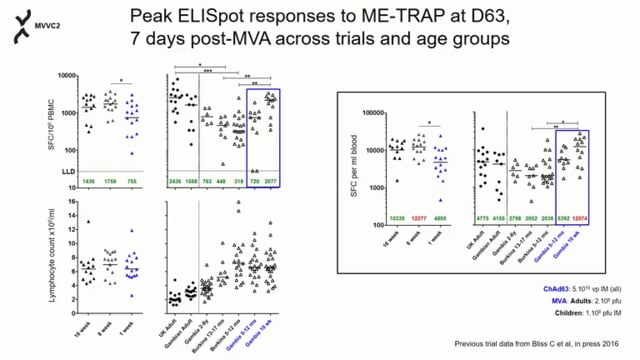
We enrolled 65 Gambian infants and neonates into 3 groups aged either 16, 8 or 1 week old at first vaccination and randomized them to receive either ME-TRAP vaccine or control. All participants received EPI vaccines according to the national programme. Safety was assessed by the description of vaccines-related adverse events including clinical assessments, biochemical and haematological tests. Immunogenicity was evaluated using anti-TRAP IgG ELISA, interferon-gamma ELISPOT and flow cytometry. Serology was performed to confirm all infants achieved protective titres to EPI vaccines.
The vaccines were well tolerated in all age groups with no vaccine-related serious adverse events. High-level TRAP specific IgG and T cell responses were generated after boosting with MVA. Particularly, CD8+ T cell responses, previously found to correlate with protection, were induced in all groups. Antibody responses to EPI vaccines remained at protective levels.
While difficult to induce in neonates with protein or polysaccharide vaccines, potent humoral and cellular immunity were generated by heterologous prime-boost immunization with ChAd63/MVA ME-TRAP in young infants and neonates. Co-administration of routine EPI vaccines did not interfere with these responses. The EPI vaccines also retained protective antibody titres following administration of the malaria vaccines, supporting further evaluation of this regimen in infants.
THEMES: Impact of Interventions | Vaccines