Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Warning: Undefined array key "file" in /var/www/vhosts/gestortectic.com/mesa.gestortectic.com/wp-content/plugins/fulltext-search/includes/wpfts_querylog.php on line 520
Last Updated: 24/05/2023
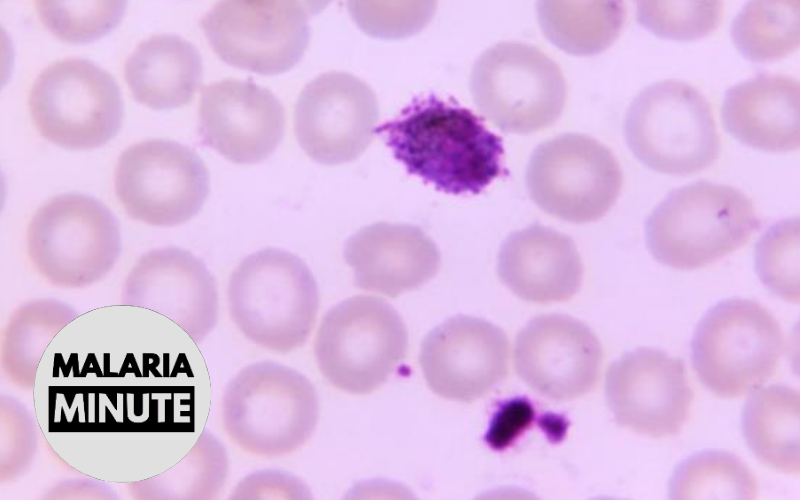
Qualification of the Plasmodium falciparum 18S rRNA biomarker for malaria field studies
Objectives
To perform biomarker testing on samples from clinical trials and utilize the data to support an expanded Context of Use for the Plasmodium falciparum 18S rRNA biomarker for malaria-endemic field studies.
Biomarker qualification is a process whereby the analytical and clinical performance of a biomarker is tested to determine its suitability for a particular Context of Use (COU). Clinical trials of malaria drugs, vaccines, and other therapeutics benefits from high quality diagnostics, but many of the existing diagnostic modalities such as rapid diagnostic tests and thick blood smear microscopy are relatively insensitive and/or operator-dependent. In contrast, molecular diagnostic biomarker testing for Plasmodium falciparum has been shown to be analytically and clinically sensitive and specific in many settings. In this project, biomarker testing will be performed on archival samples from malaria endemic site clinical trials and the data will be utilized to support an expanded Context of Use for the Plasmodium falciparum 18S rRNA biomarker for malaria-endemic field studies.
Sep 2022 — Aug 2023
$250,000