Last Updated: 17/02/2023
Investigating asymptomatic transmission of malaria parasites in the Madang province of Papua New Guinea
Objectives
To investigate the transmission of malaria parasites by individuals who are not showing symptoms of malaria within a community.
Papua New Guinea Institute of Medical Research (PNG IMR), Papua New Guinea
Papua New Guinea (PNG) is currently trying to control and curb the resurgence in malaria infections. Much of the focus is on the clinical cases with little attention placed on the asymptomatic infections. Asymptomatic malaria transmission poses a huge problem in malaria endemic and non-endemic communities as it sustains parasite transmission. These cases go undetected and are a reservoir for continuous infections. It is important to determine how much of the community are asymptomatic and potentially spreading the infection, so that proper measures can be recommended to the National Government to address these cases.
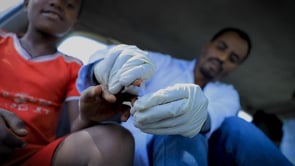
In the current study we intend to recruit 500 participants between 5 years to adult age. We will only recruit willing consented individuals. The recruitment of participants for this study will be based on malaria rapid diagnostic tests (RDT) and real-time PCR (qPCR) detection of P. falciparum and P. vivax. Between 20 to 50 mosquitoes (3-5 day olds) will be used in the DSFA. The female An. farauti mosquitoes will be transferred into a paper cup using an aspirator for transportation. The cups will be placed in an Eski cooler (Coleman Cooler, USA) and then will be transferred via a vehicle to the field site. In the DSFA, study participants will be exposing their hand to starved laboratory-reared An. farauti mosquitoes for 10 minutes. Following the feeding experiments, the unfed mosquitoes will be removed. The fully fed mosquitoes will be kept until day 7 for P. vivax and day 8-9 for P. falciparum after feeding, where they will be dissected under a special microscope and the gut will be removed and stained with 0.2% mercurochrome for 15 mins. The stained gut will then be viewed under a conventional microscope at 40X for the presence of oocysts on the gut wall.
We will also be collaborating with James Beeson and Jo-Anne Chan at the Burnet Institute to perform antibody assays. We will determine the levels of IgG and IgM isotypes, including IgG subclasses to the recombinant proteins of leading vaccine candidates, Pfs230D1M (Domain 1 which is expressed in HEK293 mammalian cells) and Pfs48/45. Further, we will evaluate the effector function of antibodies including their ability to fix complement and bind Fc receptors to mediate opsonic phagocytosis. Findings from the Beeson group have supported the contribution of these antibody mechanisms in transmission-blocking immunity. We are also intending to test P. vivax antibodies as well.
Mar 2021 — Oct 2023
$15,000