Last Updated: 29/05/2025
Mosquito immune responses and malaria transmission
Objectives
The objective of this project is to generate monoclonal antibodies to the Plasmodium faciparum surface protein Pfs47 that have transmission-blocking activity.
A single cell transcriptome analysis of >5000 individual hemocytes from Anopheles gambiae was carried out in collaboration with Dr. Oliver Billker from the Sanger Institute, that led to the identification of hemocyte populations with 7 different transcriptional profiles. Markers specific for several specific populations were validated by in situ hybridization in both circulating and tissue-associated hemocytes. Some of the markers define specific hemocyte lineages, while others reflect functional responses. For example, a subpopulation of hemocyes expressing high levels of a member of the leucine-rich repeat family is greatly increase when mosquitoes are infected with Plasmodium parasites. The manuscript describing this work in in preparation. Protein markers specific for each of these hemocyte populations are currently being expressed and making antibodies to them. The generation of these reagents is the first stepping stone to broaden our understanding hemocyte population dynamics as the hemoatopoietic system of newly emerged female mosquitoes matures and as mosquitoes respond to Plasmodium infection.
Anopheles gambiae mosquitoes that have been infected with Plasmodium mount a more effective immune response to a subsequent infection. Priming is established when Plasmodium invasion of the mosquito midgut allows contact of the gut microbiota with epithelial cells. This event is followed by a systemic release of a hemocyte differentiation factor (HDF) consisting of Lipoxin A4 bound to Evokin, a lipocalin carrier, that increases the proportion of circulating hemocytes. We show that mosquito midgut cells produce and release prostaglandin E2 (PGE2) that attracts hemocytes to the midgut surface and enhances their patrolling activity. Systemic injection of PGs recapitulates the priming response and enhances antiplasmodial immunity by triggering HDF production. Insects lack cyclooxygenases, so it has not been clear how prostaglandins are synthesized. We found that two heme peroxidases, HPX7 and HPX8, catalyze essential steps in PG biosynthesis in mosquitoes. Silencing either one of these enzymes prevents prostaglandin synthesis and disrupts the immune priming response. Mosquito midgut PGE2 release attracts hemocytes and establishes a long-lasting enhanced systemic cellular immune response to Plasmodium infection. This work was recently published in iScience.
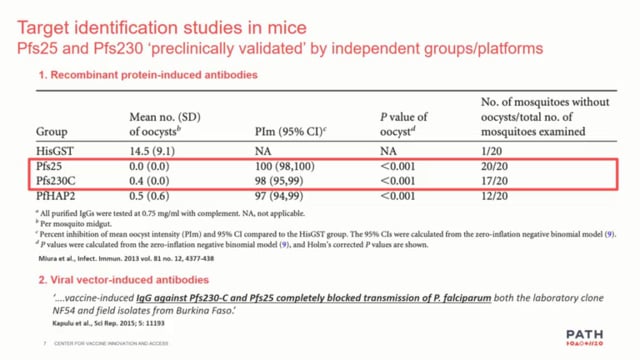
Recently Pfs47 was characterized, a protein on the surface of sexual stages and ookinetes of Plasmodium falciparum, as a malaria transmission-blocking vaccine (TBV) target. Mice immunization induced antibodies that conferred strong transmission-reducing activity (TRA) at a concentration of 200 g/mL. Here, it is sought to optimize the Pfs47 vaccine to elicit higher titers of high affinity antibodies, capable of inducing strong TRA at lower concentration. This study reports the development and evaluation of a Pfs47-based virus-like particle (VLP) vaccine generated by conjugating our 58 aa Pfs47 antigen to Acinetobacter phage AP205-VLP using the SpyCatcher:SpyTag adaptor system. AP205-Pfs47 complexes (VLP-P47) formed particles of 22 nm in diameter that reacted with polyclonal anti-Pfs47 antibodies, indicating that the antigen was accessible on the surface of the particle. Mice immunized with VLP-P47 followed by a boost with Pfs47 monomer induced significantly higher antibody titers, with higher binding affinity to Pfs47, than mice that receive two immunizations with either VLP-P47 (VLP-P47/VLP-P47) or the Pfs47 monomer (P47/P47). Purified IgG from VLP-P47/P47 mice had strong TRA (83-98%) at concentrations as low as 5 g/mL. These results indicate that conjugating the Pfs47 antigen to AP205-VLP significantly enhanced antigenicity and confirm the potential of Pfs47 as a TBV candidate. The manuscript describing this work is ready to be submitted for publication.
It has previously been shown that the Plasmodium surface protein Pfs47 is critical for malaria transmission, by allowing the parasite to evade the mosquito immune system. Ookinete invasion triggers a strong nitration response as the invaded epithelial cell undergoes apoptosis. Nitration of the midgut basal lamina, in turn, triggers the release of microvesicles by hemocytes patrolling the basal surface of the midgut. Microvesicles release promotes local activation of the mosquito complement system, a key mediator of antiplasmodial immunity that forms a complex of the ookinete surface that lyses the parasite. Pfs47 disrupts epithelial nitration and, in the absence of hemocyte-derived microvesicles, activation of the mosquito complement system is not effective. As a result, parasites survive and are transmitted to a new host. It has previously been shown that the Pfs47 is polymorphic and has a geographic population structure consistent with natural selection of Pfs47 by different anopheline species during the adaptation of P. falciparum to evolutionarily distant malaria vectors around the World. It has been proposed a lock and key model in which compatible Pfs47 haplotypes are selected by different Pfs47 receptors present in evolutionarily distant malaria vectors. The Pfs47 receptor was identified in anopheline mosquitoes and found that recombinant Pfs47 protein binds to recombinant Anopheles gambiae Pfs47 receptor (AgP47Rec) with high affinity (17 nM). AgP47Rec is the only protein in A. gambiae with two natterin-like domains, and has a clear one-to-one ortholog in other mosquito species including anophelines with 83-84 % amino acid identity. Putative orthologs are also present in more distant diptera of the same subgenus (Nematocera), such as Phlebotomus papatasi (sandfly) and Clunio marinus (a non-biting midge); while in Drosophila (subgenus Brachycera), there are several proteins with two natterin-like domains, but they are more divergent (45-58% identity). This indicates that AgP47Rec has an ancient origin in dipteran evolution. AgP47Rec protein is present in the submicrovillar region of midgut epithelial cells, the parasites surface comes in close contact with AgP47Rec and triggers local aggregation of the receptor as ookinetes penetrate the apical of the cell. We found the interaction of Pfs47 with its mosquito receptor in different vector species is consistent with the lock and key model. The manuscript describing this work is in preparation.
Oct 2007 — Oct 2019
$1.95M